当前位置:
X-MOL 学术
›
Chem. Bio. Drug Des.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Inhibiting mechanism of small molecule toward the p53‐MDM2 interaction: A molecular dynamic exploration
Chemical Biology & Drug Design ( IF 3.2 ) Pub Date : 2018-07-02 , DOI: 10.1111/cbdd.13345 Jianzhong Chen 1, 2 , Jinan Wang 2 , Laixue Pang 1 , Weiliang Zhu 2
Chemical Biology & Drug Design ( IF 3.2 ) Pub Date : 2018-07-02 , DOI: 10.1111/cbdd.13345 Jianzhong Chen 1, 2 , Jinan Wang 2 , Laixue Pang 1 , Weiliang Zhu 2
Affiliation
|
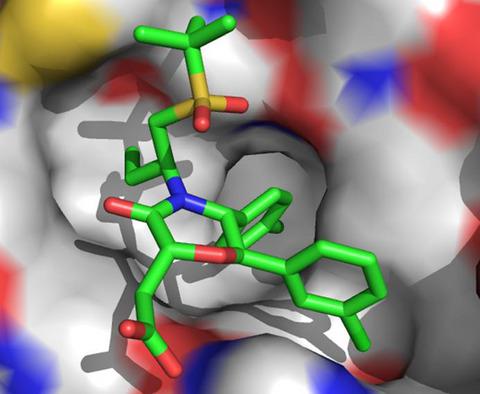
Disruption of the p53‐MDM2 interaction has been an efficient strategy to renew the function of wild‐type p53. In this work, molecular dynamic simulations, molecular mechanics–generalized Born surface area method, and principal component analysis were combined to probe interaction mechanism of inhibitors 2TZ, 2U0, 2U1, 2U5, 2U6, and 2U7 with MDM2. The rank of our current predicted binding free energies is in agreement with that of the experimental values. The results demonstrate that the introductions of thiazole and pyridine rings into 2TZ as well as the change in the orientation of inhibitors lead to the increase in the polar interactions of 2U0, 2U1, 2U5, 2U6, and 2U7 with MDM2 relative to 2TZ. The information derived from principal component analysis suggests that inhibitor bindings produce significant effect on the binding cleft of MDM2 and make the binding cleft wider and bigger so as to accommodate different type inhibitors. This study is looked forward to contributing theoretical hints for designs of potent inhibitors targeting the p53‐MDM2 interaction.
中文翻译:

小分子对p53-MDM2相互作用的抑制机制:分子动力学探索
破坏p53-MDM2相互作用一直是更新野生型p53功能的有效策略。在这项工作中,结合了分子动力学模拟,分子力学通用的Born表面积法和主成分分析,以探究抑制剂2TZ,2U0、2U1、2U5、2U6和2U7与MDM2的相互作用机理。我们目前预测的结合自由能的等级与实验值的等级一致。结果表明,相对于2TZ,噻唑和吡啶环引入2TZ以及抑制剂取向的改变导致2U0、2U1、2U5、2U6和2U7与MDM2的极性相互作用增加。从主成分分析得出的信息表明,抑制剂结合对MDM2的结合裂隙产生显着影响,并使结合裂隙变宽和变大,以适应不同类型的抑制剂。这项研究期待为靶向p53-MDM2相互作用的有效抑制剂的设计提供理论指导。
更新日期:2018-07-02
中文翻译:

小分子对p53-MDM2相互作用的抑制机制:分子动力学探索
破坏p53-MDM2相互作用一直是更新野生型p53功能的有效策略。在这项工作中,结合了分子动力学模拟,分子力学通用的Born表面积法和主成分分析,以探究抑制剂2TZ,2U0、2U1、2U5、2U6和2U7与MDM2的相互作用机理。我们目前预测的结合自由能的等级与实验值的等级一致。结果表明,相对于2TZ,噻唑和吡啶环引入2TZ以及抑制剂取向的改变导致2U0、2U1、2U5、2U6和2U7与MDM2的极性相互作用增加。从主成分分析得出的信息表明,抑制剂结合对MDM2的结合裂隙产生显着影响,并使结合裂隙变宽和变大,以适应不同类型的抑制剂。这项研究期待为靶向p53-MDM2相互作用的有效抑制剂的设计提供理论指导。











































 京公网安备 11010802027423号
京公网安备 11010802027423号