当前位置:
X-MOL 学术
›
New J. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Chiral copper-salen complex grafted over functionalized mesoporous silica as an efficient catalyst for asymmetric Henry reactions and synthesis of the potent drug (R)-isoproterenol†
New Journal of Chemistry ( IF 2.7 ) Pub Date : 2018-06-07 00:00:00 , DOI: 10.1039/c8nj01745j Mita Halder 1, 2, 3, 4 , Piyali Bhanja 4, 5, 6 , Md. Mominul Islam 1, 4, 7, 8 , Asim Bhaumik 4, 5, 6 , Sk. Manirul Islam 1, 4, 7, 8
New Journal of Chemistry ( IF 2.7 ) Pub Date : 2018-06-07 00:00:00 , DOI: 10.1039/c8nj01745j Mita Halder 1, 2, 3, 4 , Piyali Bhanja 4, 5, 6 , Md. Mominul Islam 1, 4, 7, 8 , Asim Bhaumik 4, 5, 6 , Sk. Manirul Islam 1, 4, 7, 8
Affiliation
|
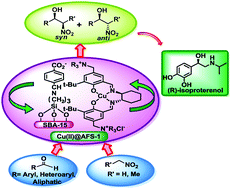
Synthesis of enantiomerically pure drug molecules using functionalized mesoporous materials bearing a chiral moiety is a longstanding goal in heterogeneous catalysis. Herein, we report an efficient and enantioselective one-pot Henry reaction over a highly ordered functionalized mesoporous silica-supported chiral copper-salen catalyst, Cu(II)@AFS-1, in DCM at RT. The catalyst was characterized by UV-Vis, FT-IR spectroscopy, PXRD, N2 adsorption/desorption, HR-TEM, EPR, and thermogravimetric and elemental analyses. The diastereoselective Henry reaction of nitroethane was carried out by using this catalyst to obtain good yields (up to 96%) and ee (up to 95%) of β-nitroalcohols with acceptable dr (3.2 : 1). Additionally, the enantiomerically pure drug (R)-(−)-isoproterenol can be synthesized through the asymmetric Henry reaction of 3,4-dimethoxybenzaldehyde over this chiral mesoporous Cu-catalyst as a key step.
中文翻译:

手性铜-salen络合物接枝在功能化介孔二氧化硅上,作为不对称亨利反应和强效药物(R)-异丙肾上腺素的合成的有效催化剂†
使用带有手性部分的功能化介孔材料合成对映体纯净的药物分子是多相催化的长期目标。在本文中,我们报告了在室温下于DCM中对高度有序的官能化介孔二氧化硅负载的手性铜salen催化剂Cu(II)@ AFS-1进行的高效且对映选择性的一锅亨利反应。通过UV-Vis,FT-IR光谱,PXRD,N 2吸附/解吸,HR-TEM,EPR以及热重和元素分析对催化剂进行了表征。使用该催化剂进行硝基乙烷的非对映选择性亨利反应,可获得良好的产率(高达96%)和ee(高达95%)的β-硝基醇,且其dr(3.2:1)可接受。另外,对映体纯药物(R作为关键步骤,可以通过3,4-二甲氧基苯甲醛在该手性介孔Cu催化剂上的不对称亨利反应合成)-(-)-异丙基肾上腺素。
更新日期:2018-06-07
中文翻译:

手性铜-salen络合物接枝在功能化介孔二氧化硅上,作为不对称亨利反应和强效药物(R)-异丙肾上腺素的合成的有效催化剂†
使用带有手性部分的功能化介孔材料合成对映体纯净的药物分子是多相催化的长期目标。在本文中,我们报告了在室温下于DCM中对高度有序的官能化介孔二氧化硅负载的手性铜salen催化剂Cu(II)@ AFS-1进行的高效且对映选择性的一锅亨利反应。通过UV-Vis,FT-IR光谱,PXRD,N 2吸附/解吸,HR-TEM,EPR以及热重和元素分析对催化剂进行了表征。使用该催化剂进行硝基乙烷的非对映选择性亨利反应,可获得良好的产率(高达96%)和ee(高达95%)的β-硝基醇,且其dr(3.2:1)可接受。另外,对映体纯药物(R作为关键步骤,可以通过3,4-二甲氧基苯甲醛在该手性介孔Cu催化剂上的不对称亨利反应合成)-(-)-异丙基肾上腺素。











































 京公网安备 11010802027423号
京公网安备 11010802027423号