当前位置:
X-MOL 学术
›
Adv. Synth. Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Oxidative Radical Cyclization of N‐methyl‐N‐arylpropiolamide to Isatins via Cleavage of the Carbon‐carbon Triple Bond
Advanced Synthesis & Catalysis ( IF 4.4 ) Pub Date : 2018-07-08 , DOI: 10.1002/adsc.201800592 Yan-Yan Liao 1 , Yong-Chao Gao 1 , Wenxu Zheng 1 , Ri-Yuan Tang 1, 2
Advanced Synthesis & Catalysis ( IF 4.4 ) Pub Date : 2018-07-08 , DOI: 10.1002/adsc.201800592 Yan-Yan Liao 1 , Yong-Chao Gao 1 , Wenxu Zheng 1 , Ri-Yuan Tang 1, 2
Affiliation
|
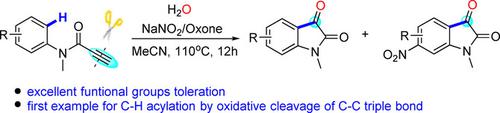
A radical cyclization of N‐methyl‐N‐arylpropiolamide to isatins via an oxidative cleavage of a carbon‐carbon triple bond has been developed. In the presence of oxone and NaNO2, a variety of N‐methyl‐N‐arylpropiolamides were smoothly transformed into isatins. A nitration reaction proceeded along with the oxidative cyclization; both nitrated and non‐nitrated isatins were obtained in a one‐pot reaction with moderate to good total yields. This is the first example for the synthesis of isatins via the oxidative radical cleavage of a carbon‐carbon triple bond.
中文翻译:

N-甲基-N-芳基丙醇酰胺通过碳-碳三键的裂解被氧化成Isatins的氧化自由基环化。
已开发出通过碳-碳三键的氧化裂解将N-甲基-N-芳基丙醇酰胺自由基环化为靛红的方法。在存在丙酮和NaNO 2的情况下,各种N-甲基-N-芳基丙酰胺都可以平稳地转化为靛红。硝化反应与氧化环化一起进行。一锅反应可同时获得硝化和未硝化的靛红,总收率中等至良好。这是通过碳-碳三键的氧化自由基裂解合成Isatin的第一个例子。
更新日期:2018-07-08
中文翻译:

N-甲基-N-芳基丙醇酰胺通过碳-碳三键的裂解被氧化成Isatins的氧化自由基环化。
已开发出通过碳-碳三键的氧化裂解将N-甲基-N-芳基丙醇酰胺自由基环化为靛红的方法。在存在丙酮和NaNO 2的情况下,各种N-甲基-N-芳基丙酰胺都可以平稳地转化为靛红。硝化反应与氧化环化一起进行。一锅反应可同时获得硝化和未硝化的靛红,总收率中等至良好。这是通过碳-碳三键的氧化自由基裂解合成Isatin的第一个例子。










































 京公网安备 11010802027423号
京公网安备 11010802027423号