Acta Biomaterialia ( IF 9.4 ) Pub Date : 2018-06-07 , DOI: 10.1016/j.actbio.2018.06.007 Minh K Nguyen 1 , Oju Jeon 1 , Phuong N Dang 1 , Cong T Huynh 1 , Davood Varghai 2 , Hooman Riazi 2 , Alexandra McMillan 1 , Samuel Herberg 1 , Eben Alsberg 3
|
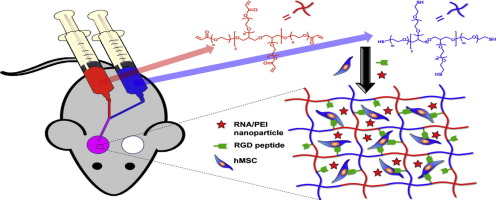
RNA interference (RNAi) may be an effective and valuable tool for promoting the growth of functional tissue, as short interfering RNA (siRNA) and microRNA (miRNA) can block the expression of genes that have negative effects on tissue regeneration. Our group has recently reported that the localized and sustained presentation of siRNA against noggin (siNoggin) and miRNA-20a from in situ forming poly(ethylene glycol) (PEG) hydrogels enhanced osteogenic differentiation of encapsulated human bone marrow-derived mesenchymal stem cells (hMSCs). Here, the capacity of the hydrogel system to accelerate bone formation in a rat calvarial bone defect model is presented. After 12 weeks post-implantation, the hydrogels containing encapsulated hMSCs and miRNA-20a resulted in more bone formation in the defects than the hydrogels containing hMSCs without siRNA or with negative control siRNA. This localized and sustained RNA interfering molecule delivery system may provide an excellent platform for healing bony defects and other tissues.
Statement of Significance
Delivery of RNAi molecules may be a valuable strategy to guide cell behavior for tissue engineering applications, but to date there have been no reports of a biomaterial system capable of both encapsulation of cells and controlled delivery of incorporated RNA. Here, we present PEG hydrogels that form in situ via Michael type reaction, and that permit encapsulation of hMSCs and the concomitant controlled delivery of siNoggin and/or miRNA-20a. These RNAs were chosen to suppress noggin, a BMP-2 antagonist, and/or PPAR-γ, a negative regulator of BMP-2-mediated osteogenesis, and therefore promote osteogenic differentiation of hMSCs and subsequent bone repair in critical-sized rat calvarial defects. Simultaneous delivery of hMSCs and miRNA-20a enhanced repair of these defects compared to hydrogels containing hMSCs without siRNA or with negative control siRNA. This in situ forming PEG hydrogel system offers an exciting platform for healing critical-sized bone defects by localized, controlled delivery of RNAi molecules to encapsulated hMSCs and surrounding cells.
中文翻译:

原位形成可生物降解水凝胶的 RNA 干扰分子递送用于增强大鼠颅骨骨缺损的骨形成
RNA干扰(RNAi)可能是促进功能组织生长的有效且有价值的工具,因为短干扰RNA(siRNA)和微小RNA(miRNA)可以阻断对组织再生有负面影响的基因的表达。我们的小组最近报道,来自原位形成聚乙二醇(PEG)水凝胶的针对noggin(siNoggin)和miRNA-20a的siRNA的局部和持续呈递增强了封装的人骨髓源性间充质干细胞(hMSC)的成骨分化。 )。在此,介绍了水凝胶系统在大鼠颅骨骨缺损模型中加速骨形成的能力。植入后12周后,与含有不含siRNA或含有阴性对照siRNA的hMSC的水凝胶相比,含有封装的hMSC和miRNA-20a的水凝胶导致缺损处更多的骨形成。这种局部且持续的RNA干扰分子递送系统可以为治疗骨缺损和其他组织提供一个极好的平台。
重要性声明
RNAi分子的递送可能是组织工程应用中指导细胞行为的一种有价值的策略,但迄今为止,还没有关于能够封装细胞和控制递送掺入的RNA的生物材料系统的报道。在这里,我们提出了通过迈克尔型反应原位形成的 PEG 水凝胶,它允许封装 hMSC 并伴随 siNoggin 和/或 miRNA-20a 的受控递送。选择这些 RNA 来抑制 noggin(一种 BMP-2 拮抗剂)和/或 PPAR-γ(BMP-2 介导的成骨的负调节因子),从而促进 hMSC 的成骨分化以及随后临界尺寸大鼠颅骨缺损的骨修复。与含有不含 siRNA 或含有阴性对照 siRNA 的 hMSC 的水凝胶相比,同时递送 hMSC 和 miRNA-20a 增强了这些缺陷的修复。这种原位形成的 PEG 水凝胶系统提供了一个令人兴奋的平台,通过将 RNAi 分子局部、受控地递送至封装的 hMSC 和周围细胞,来治愈临界尺寸的骨缺损。











































 京公网安备 11010802027423号
京公网安备 11010802027423号