当前位置:
X-MOL 学术
›
Biochemistry
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
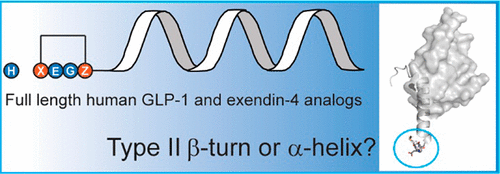
α-Helix or β-Turn? An Investigation into N-Terminally Constrained Analogues of Glucagon-like Peptide 1 (GLP-1) and Exendin-4
Biochemistry ( IF 2.9 ) Pub Date : 2018-06-07 00:00:00 , DOI: 10.1021/acs.biochem.8b00105 Alberto Oddo 1 , Sofia Mortensen 1 , Henning Thøgersen 1 , Leonardo De Maria 1 , Stephanie Hennen 1 , James N. McGuire 1 , Jacob Kofoed 1 , Lars Linderoth 1 , Steffen Reedtz-Runge 1
Biochemistry ( IF 2.9 ) Pub Date : 2018-06-07 00:00:00 , DOI: 10.1021/acs.biochem.8b00105 Alberto Oddo 1 , Sofia Mortensen 1 , Henning Thøgersen 1 , Leonardo De Maria 1 , Stephanie Hennen 1 , James N. McGuire 1 , Jacob Kofoed 1 , Lars Linderoth 1 , Steffen Reedtz-Runge 1
Affiliation
|
Peptide agonists acting on the glucagon-like peptide 1 receptor (GLP-1R) promote glucose-dependent insulin release and therefore represent important therapeutic agents for type 2 diabetes (T2D). Previous data indicated that an N-terminal type II β-turn motif might be an important feature for agonists acting on the GLP-1R. In contrast, recent publications reporting the structure of the full-length GLP-1R have shown the N-terminus of receptor-bound agonists in an α-helical conformation. To reconcile these conflicting results, we prepared N-terminally constrained analogues of glucagon-like peptide 1 (GLP-1) and exendin-4 and evaluated their receptor affinity and functionality in vitro; we then examined their crystal structures in complex with the extracellular domain of the GLP-1R and used molecular modeling and molecular dynamics simulations for further investigations. We report that the peptides’ N-termini in all determined crystal structures adopted a type II β-turn conformation, but in vitro potency varied several thousand-fold across the series. Potency correlated better with α-helicity in our computational model, although we have found that the energy barrier between the two mentioned conformations is low in our most potent analogues and the flexibility of the N-terminus is highlighted by the dynamics simulations.
中文翻译:

α-螺旋或β-转弯?胰高血糖素样肽1(GLP-1)和Exendin-4的N末端约束类似物的研究。
作用于胰高血糖素样肽1受体(GLP-1R)的肽激动剂可促进葡萄糖依赖性胰岛素的释放,因此代表了2型糖尿病(T2D)的重要治疗剂。先前的数据表明,对于作用于GLP-1R的激动剂,N端II型β-转角基序可能是一个重要特征。相反,报道全长GLP-1R的结构的最新出版物显示了受体结合激动剂的N-末端呈α-螺旋构象。为了调和这些矛盾的结果,我们制备了胰高血糖素样肽1(GLP-1)和exendin-4的N末端受限类似物,并在体外评估了它们的受体亲和力和功能; 然后,我们检查了其晶体结构与GLP-1R胞外结构域的复合情况,并使用分子模型和分子动力学模拟进行了进一步的研究。我们报告说,在所有确定的晶体结构中,肽的N末端均采用II型β-转角构象,但在整个系列中,体外效价变化了数千倍。在我们的计算模型中,效价与α螺旋度的相关性更好,尽管我们已经发现,在我们最有效的类似物中,上述两个构象之间的能垒很低,并且动力学模拟突出了N末端的灵活性。
更新日期:2018-06-07
中文翻译:

α-螺旋或β-转弯?胰高血糖素样肽1(GLP-1)和Exendin-4的N末端约束类似物的研究。
作用于胰高血糖素样肽1受体(GLP-1R)的肽激动剂可促进葡萄糖依赖性胰岛素的释放,因此代表了2型糖尿病(T2D)的重要治疗剂。先前的数据表明,对于作用于GLP-1R的激动剂,N端II型β-转角基序可能是一个重要特征。相反,报道全长GLP-1R的结构的最新出版物显示了受体结合激动剂的N-末端呈α-螺旋构象。为了调和这些矛盾的结果,我们制备了胰高血糖素样肽1(GLP-1)和exendin-4的N末端受限类似物,并在体外评估了它们的受体亲和力和功能; 然后,我们检查了其晶体结构与GLP-1R胞外结构域的复合情况,并使用分子模型和分子动力学模拟进行了进一步的研究。我们报告说,在所有确定的晶体结构中,肽的N末端均采用II型β-转角构象,但在整个系列中,体外效价变化了数千倍。在我们的计算模型中,效价与α螺旋度的相关性更好,尽管我们已经发现,在我们最有效的类似物中,上述两个构象之间的能垒很低,并且动力学模拟突出了N末端的灵活性。



























 京公网安备 11010802027423号
京公网安备 11010802027423号