Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
EMI1 switches from being a substrate to an inhibitor of APC/CCDH1 to start the cell cycle
Nature ( IF 50.5 ) Pub Date : 2018-06-01 , DOI: 10.1038/s41586-018-0199-7 Steven D Cappell 1, 2 , Kevin G Mark 3, 4 , Damien Garbett 1 , Lindsey R Pack 1 , Michael Rape 3, 4 , Tobias Meyer 1
Nature ( IF 50.5 ) Pub Date : 2018-06-01 , DOI: 10.1038/s41586-018-0199-7 Steven D Cappell 1, 2 , Kevin G Mark 3, 4 , Damien Garbett 1 , Lindsey R Pack 1 , Michael Rape 3, 4 , Tobias Meyer 1
Affiliation
|
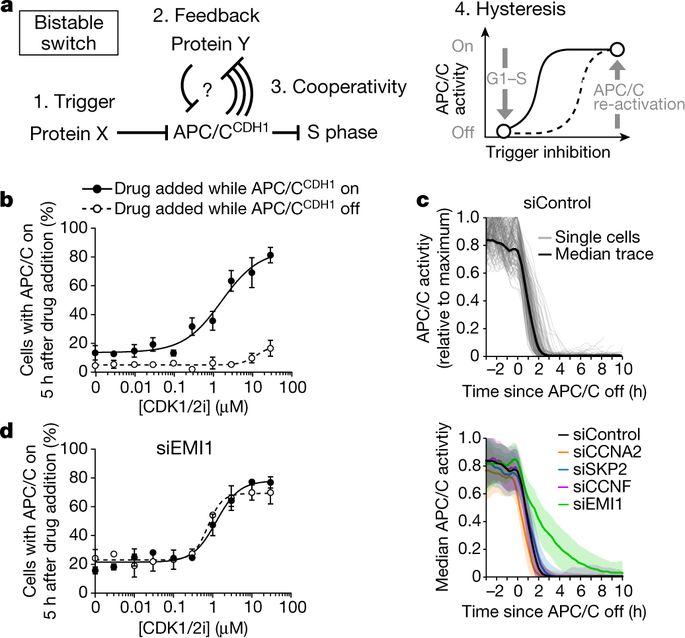
Mammalian cells integrate mitogen and stress signalling before the end of G1 phase to determine whether or not they enter the cell cycle1–4. Before cells can replicate their DNA in S phase, they have to activate cyclin-dependent kinases (CDKs), induce an E2F transcription program and inactivate the anaphase-promoting complex (APC/CCDH1, also known as the cyclosome), which is an E3 ubiquitin ligase that contains the co-activator CDH1 (also known as FZR, encoded by FZR1). It was recently shown that stress can return cells to quiescence after CDK2 activation and E2F induction but not after inactivation of APC/CCDH1, which suggests that APC/CCDH1 inactivation is the point of no return for cell-cycle entry3. Rapid inactivation of APC/CCDH1 requires early mitotic inhibitor 1 (EMI1)3,5, but the molecular mechanism that controls this cell-cycle commitment step is unknown. Here we show using human cell models that cell-cycle commitment is mediated by an EMI1–APC/CCDH1 dual-negative feedback switch, in which EMI1 is both a substrate and an inhibitor of APC/CCDH1. The inactivation switch triggers a transition between a state with low EMI1 levels and high APC/CCDH1 activity during G1 and a state with high EMI1 levels and low APC/CCDH1 activity during S and G2. Cell-based analysis, in vitro reconstitution and modelling data show that the underlying dual-negative feedback is bistable and represents a robust irreversible switch. Our study suggests that mammalian cells commit to the cell cycle by increasing CDK2 activity and EMI1 mRNA expression to trigger a one-way APC/CCDH1 inactivation switch that is mediated by EMI1 transitioning from acting as a substrate of APC/CCDH1 to being an inhibitor of APC/CCDH1.The transition between early mitotic inhibitor 1 acting as a substrate of the APC/C and as an inhibitor of the same complex results in an irreversible switch that mediates human cell-cycle commitment.
中文翻译:

EMI1 从底物转变为 APC/CCDH1 的抑制剂以启动细胞周期
哺乳动物细胞在 G1 期结束前整合有丝分裂原和应激信号,以确定它们是否进入细胞周期 1-4。在细胞可以在 S 期复制其 DNA 之前,它们必须激活细胞周期蛋白依赖性激酶 (CDK),诱导 E2F 转录程序并使后期促进复合物 (APC/CCDH1,也称为环体) 失活,这是一种 E3含有共激活因子 CDH1(也称为 FZR,由 FZR1 编码)的泛素连接酶。最近表明,在 CDK2 激活和 E2F 诱导后,压力可以使细胞恢复静止,但在 APC/CCDH1 失活后不能,这表明 APC/CCDH1 失活是进入细胞周期的不归路点。APC/CCDH1 的快速失活需要早期有丝分裂抑制剂 1 (EMI1)3,5, 但控制这一细胞周期承诺步骤的分子机制尚不清楚。在这里,我们使用人类细胞模型显示细胞周期承诺是由 EMI1-APC/CCDH1 双负反馈开关介导的,其中 EMI1 既是 APC/CCDH1 的底物又是抑制剂。失活开关触发在 G1 期间具有低 EMI1 水平和高 APC/CCDH1 活动的状态与在 S 和 G2 期间具有高 EMI1 水平和低 APC/CCDH1 活动的状态之间的转换。基于细胞的分析、体外重组和建模数据表明,潜在的双重负反馈是双稳态的,代表了一个强大的不可逆开关。
更新日期:2018-06-01
中文翻译:

EMI1 从底物转变为 APC/CCDH1 的抑制剂以启动细胞周期
哺乳动物细胞在 G1 期结束前整合有丝分裂原和应激信号,以确定它们是否进入细胞周期 1-4。在细胞可以在 S 期复制其 DNA 之前,它们必须激活细胞周期蛋白依赖性激酶 (CDK),诱导 E2F 转录程序并使后期促进复合物 (APC/CCDH1,也称为环体) 失活,这是一种 E3含有共激活因子 CDH1(也称为 FZR,由 FZR1 编码)的泛素连接酶。最近表明,在 CDK2 激活和 E2F 诱导后,压力可以使细胞恢复静止,但在 APC/CCDH1 失活后不能,这表明 APC/CCDH1 失活是进入细胞周期的不归路点。APC/CCDH1 的快速失活需要早期有丝分裂抑制剂 1 (EMI1)3,5, 但控制这一细胞周期承诺步骤的分子机制尚不清楚。在这里,我们使用人类细胞模型显示细胞周期承诺是由 EMI1-APC/CCDH1 双负反馈开关介导的,其中 EMI1 既是 APC/CCDH1 的底物又是抑制剂。失活开关触发在 G1 期间具有低 EMI1 水平和高 APC/CCDH1 活动的状态与在 S 和 G2 期间具有高 EMI1 水平和低 APC/CCDH1 活动的状态之间的转换。基于细胞的分析、体外重组和建模数据表明,潜在的双重负反馈是双稳态的,代表了一个强大的不可逆开关。











































 京公网安备 11010802027423号
京公网安备 11010802027423号