当前位置:
X-MOL 学术
›
Bioconjugate Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Site-Specific Incorporation of Selenocysteine by Genetic Encoding as a Photocaged Unnatural Amino Acid
Bioconjugate Chemistry ( IF 4.0 ) Pub Date : 2018-06-06 00:00:00 , DOI: 10.1021/acs.bioconjchem.8b00254 Adarshi P. Welegedara 1 , Luke A. Adams 2 , Thomas Huber 1 , Bim Graham 2 , Gottfried Otting 1
Bioconjugate Chemistry ( IF 4.0 ) Pub Date : 2018-06-06 00:00:00 , DOI: 10.1021/acs.bioconjchem.8b00254 Adarshi P. Welegedara 1 , Luke A. Adams 2 , Thomas Huber 1 , Bim Graham 2 , Gottfried Otting 1
Affiliation
|
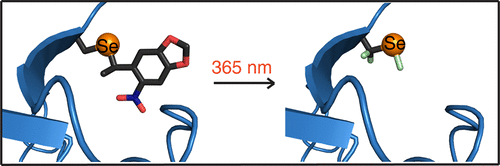
Selenocysteine (Sec) is a naturally occurring amino acid that is also referred to as the 21st amino acid. Site-specific incorporation of Sec into proteins is attractive, because the reactivity of a selenol group exceeds that of a thiol group and thus allows site-specific protein modifications. It is incorporated into proteins by an unusual enzymatic mechanism which, in E. coli and other organisms, involves the recognition of a selenocysteine insertion sequence (SECIS) in the mRNA of the target protein. Reengineering of the natural machinery for Sec incorporation at arbitrary sites independent of SECIS elements, however, is challenging. Here we demonstrate an alternative route, whereby a photocaged selenocysteine (PSc) is incorporated as an unnatural amino acid in response to an amber stop codon, using a mutant Methanosarcina mazei pyrrolysyl-tRNA synthetase, Mm PCC2RS, and its cognate tRNACUA. Following decaging by UV irradiation, proteins synthesized with PSc are readily tagged, e.g., with NMR probes to study ligand binding by NMR spectroscopy. The approach provides a facile route for genetically encoded Sec incorporation. It allows the production of pure selenoproteins and the Sec residue enables site-specific covalent protein modification with reagents that would usually react first with naturally occurring cysteine residues. The much greater reactivity of Sec residues allows their selective alkylation in the presence of highly solvent-exposed cysteine residues.
中文翻译:

通过遗传编码为光笼笼的非天然氨基酸的Selenocysteine的站点特定纳入。
硒代半胱氨酸(Sec)是天然存在的氨基酸,也称为21st氨基酸。Sec在蛋白质中的位点特异性结合是有吸引力的,因为硒醇基团的反应性超过了硫醇基团的反应性,因此可以进行位点特异性蛋白质修饰。它通过一种异常的酶促机制掺入蛋白质中,这种机制在大肠杆菌中和其他生物,涉及识别目标蛋白的mRNA中的硒代半胱氨酸插入序列(SECIS)。然而,如何在不依赖SECIS元素的任意地点对Sec合并的天然机械进行重新设计具有挑战性。在这里,我们展示了一种替代途径,其中使用突变型甲烷单孢甲烷甲烷吡咯烷基-tRNA合成酶Mm PCC2RS及其关联的tRNA CUA来将光笼笼中的硒代半胱氨酸(PSc)作为非天然氨基酸掺入琥珀终止密码子。在通过紫外线辐照降解之后,可以容易地标记用PSc合成的蛋白质,例如用NMR探针标记,以通过NMR光谱研究配体结合。该方法为遗传编码的Sec掺入提供了简便的途径。它可以产生纯净的硒蛋白,而Sec残基则可以用通常会先与天然半胱氨酸残基起反应的试剂进行位点特异性共价蛋白修饰。Sec残基的更高的反应性可使其在存在高度溶剂暴露的半胱氨酸残基的情况下进行选择性烷基化。
更新日期:2018-06-06
中文翻译:

通过遗传编码为光笼笼的非天然氨基酸的Selenocysteine的站点特定纳入。
硒代半胱氨酸(Sec)是天然存在的氨基酸,也称为21st氨基酸。Sec在蛋白质中的位点特异性结合是有吸引力的,因为硒醇基团的反应性超过了硫醇基团的反应性,因此可以进行位点特异性蛋白质修饰。它通过一种异常的酶促机制掺入蛋白质中,这种机制在大肠杆菌中和其他生物,涉及识别目标蛋白的mRNA中的硒代半胱氨酸插入序列(SECIS)。然而,如何在不依赖SECIS元素的任意地点对Sec合并的天然机械进行重新设计具有挑战性。在这里,我们展示了一种替代途径,其中使用突变型甲烷单孢甲烷甲烷吡咯烷基-tRNA合成酶Mm PCC2RS及其关联的tRNA CUA来将光笼笼中的硒代半胱氨酸(PSc)作为非天然氨基酸掺入琥珀终止密码子。在通过紫外线辐照降解之后,可以容易地标记用PSc合成的蛋白质,例如用NMR探针标记,以通过NMR光谱研究配体结合。该方法为遗传编码的Sec掺入提供了简便的途径。它可以产生纯净的硒蛋白,而Sec残基则可以用通常会先与天然半胱氨酸残基起反应的试剂进行位点特异性共价蛋白修饰。Sec残基的更高的反应性可使其在存在高度溶剂暴露的半胱氨酸残基的情况下进行选择性烷基化。











































 京公网安备 11010802027423号
京公网安备 11010802027423号