当前位置:
X-MOL 学术
›
J. Chem. Theory Comput.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
A Funneled Conformational Landscape Governs Flavivirus Fusion Peptide Interaction with Lipid Membranes
Journal of Chemical Theory and Computation ( IF 5.7 ) Pub Date : 2018-06-06 00:00:00 , DOI: 10.1021/acs.jctc.8b00438 Jan K. Marzinek 1 , Nirmalya Bag , Roland G. Huber 1 , Daniel A. Holdbrook 1 , Thorsten Wohland , Chandra S. Verma 1, 2 , Peter J. Bond 1
Journal of Chemical Theory and Computation ( IF 5.7 ) Pub Date : 2018-06-06 00:00:00 , DOI: 10.1021/acs.jctc.8b00438 Jan K. Marzinek 1 , Nirmalya Bag , Roland G. Huber 1 , Daniel A. Holdbrook 1 , Thorsten Wohland , Chandra S. Verma 1, 2 , Peter J. Bond 1
Affiliation
|
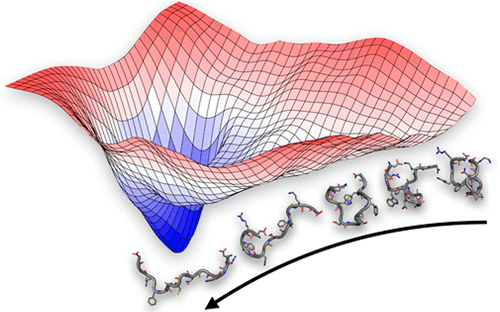
During host cell infection by flaviviruses such as dengue and Zika, acidic pH within the endosome triggers a conformational change in the envelope protein on the outer surface of the virion. This results in exposure of the ∼15 residue fusion peptide (FP) region, freeing it to induce fusion between the viral and endosomal membranes. A better understanding of the conformational dynamics of the FP in the presence of membranes, and the basis for its selectivity for anionic lipid species present within the endosome, would facilitate its therapeutic targeting with antiviral drugs and antibodies. In this work, multiscale modeling, simulations, and free energy calculations (including a total of ∼75 μs of atomic-resolution sampling), combined with imaging total internal reflection fluorescence correlation spectroscopy experiments, were employed to investigate the mechanisms of interaction of FP variants with lipid bilayers. Wild-type FPs (in the presence or absence of a fluorescein isothiocyanate tag) were shown to possess a funneled conformational landscape governing their exit from solvent and penetration into the lipid phase and to exhibit an electrostatically favored >2-fold affinity for membranes containing anionic species over purely zwitterionic ones. Conversely, the landscape was abolished in a nonfunctional point mutant, leading to a 2-fold drop in host membrane affinity. Collectively, our data reveal how the highly conserved flavivirus FP has evolved to funnel its conformational space toward a maximally fusogenic state anchored within the endosomal membrane. Therapeutically targeting the accessible ensemble of FP conformations may represent a new, rational strategy for blocking viral infection.
中文翻译:

漏斗的构象景观控制黄病毒融合肽与脂质膜的相互作用。
在黄病毒如登革热和寨卡病毒感染宿主细胞的过程中,内体中的酸性pH触发了病毒体外表面包膜蛋白的构象变化。这会导致〜15个残基融合肽(FP)区暴露,使其游离以诱导病毒膜与内体膜之间的融合。对FP在膜存在下的构象动力学的更好理解,以及对内体中存在的阴离子脂质种类的选择性基础,将有助于其通过抗病毒药物和抗体进行治疗性靶向。在这项工作中,多尺度建模,模拟和自由能计算(包括总共约75μs的原子分辨率采样)与成像全内反射荧光相关光谱实验相结合,被用来研究FP变异体与脂质双层相互作用的机制。野生型FP(存在或不存在异硫氰酸荧光素标签的情况下)显示出漏斗状的构象格局,支配着它们从溶剂中脱出并渗透到脂质相中,并且对包含阴离子的膜表现出静电吸附> 2倍的亲和力种类超过纯两性离子种类。相反,在非功能性点突变体中,景观被消除,导致宿主膜亲和力下降了2倍。总的来说,我们的数据揭示了高度保守的黄病毒FP如何进化使其构象空间朝着锚定在内体膜内的最大融合状态扩散。治疗性地针对FP构象的可访问集合可能代表了一种新的,
更新日期:2018-06-06
中文翻译:

漏斗的构象景观控制黄病毒融合肽与脂质膜的相互作用。
在黄病毒如登革热和寨卡病毒感染宿主细胞的过程中,内体中的酸性pH触发了病毒体外表面包膜蛋白的构象变化。这会导致〜15个残基融合肽(FP)区暴露,使其游离以诱导病毒膜与内体膜之间的融合。对FP在膜存在下的构象动力学的更好理解,以及对内体中存在的阴离子脂质种类的选择性基础,将有助于其通过抗病毒药物和抗体进行治疗性靶向。在这项工作中,多尺度建模,模拟和自由能计算(包括总共约75μs的原子分辨率采样)与成像全内反射荧光相关光谱实验相结合,被用来研究FP变异体与脂质双层相互作用的机制。野生型FP(存在或不存在异硫氰酸荧光素标签的情况下)显示出漏斗状的构象格局,支配着它们从溶剂中脱出并渗透到脂质相中,并且对包含阴离子的膜表现出静电吸附> 2倍的亲和力种类超过纯两性离子种类。相反,在非功能性点突变体中,景观被消除,导致宿主膜亲和力下降了2倍。总的来说,我们的数据揭示了高度保守的黄病毒FP如何进化使其构象空间朝着锚定在内体膜内的最大融合状态扩散。治疗性地针对FP构象的可访问集合可能代表了一种新的,











































 京公网安备 11010802027423号
京公网安备 11010802027423号