当前位置:
X-MOL 学术
›
J. Phys. Chem. B
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Quantifying the Effects of Hydrogen Bonding on Nitrile Frequencies in GFP: Beyond Solvent Exposure
The Journal of Physical Chemistry B ( IF 2.8 ) Pub Date : 2018-06-25 , DOI: 10.1021/acs.jpcb.8b03907 Jeremy T. First 1 , Joshua D. Slocum 1 , Lauren J. Webb 1
The Journal of Physical Chemistry B ( IF 2.8 ) Pub Date : 2018-06-25 , DOI: 10.1021/acs.jpcb.8b03907 Jeremy T. First 1 , Joshua D. Slocum 1 , Lauren J. Webb 1
Affiliation
|
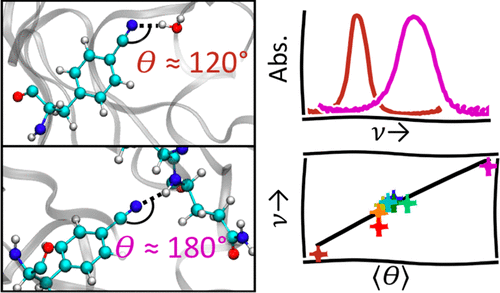
Vibrational spectroscopy is a powerful tool for characterizing the complex noncovalent interactions that arise in biological systems. The nitrile stretching frequency has proven to be a particularly convenient biological probe, but the interpretation of nitrile spectroscopy is complicated by its sensitivity to local hydrogen bonding interactions. This often inhibits the straightforward interpretation of nitrile spectra by requiring knowledge of the molecular-level details of the local environment surrounding the probe. While the effect of hydrogen bonds on nitrile frequencies has been well-documented for small molecules in solution, there have been relatively few studies of these effects in a complex protein system. To address this, we introduced a nitrile probe at nine locations throughout green fluorescent protein (GFP) and compared the mean vibrational frequency of each probe to the specific hydrogen bonding geometries observed in molecular dynamics (MD) simulations. We show that a continuum of hydrogen bonding angles is found depending on the particular location of each nitrile, and that the differences in these angles account for the differences in the measured nitrile frequency. We further observed that the temperature dependence of the nitrile frequencies (measured as a frequency–temperature line slope, FTLS) was a good indicator of the hydrogen bonding interactions of the probe, even in scenarios where the nitrile was involved in complex and restricted hydrogen bonds, both from protein and from water. While constant offsets to the nitrile frequency to all hydrogen bonding environments have been applied before to interpret shifts in nitrile frequency, we show that this is insufficient in systems where the hydrogen bonds may be restricted by the surrounding medium. However, the strength of the observed correlation between nitrile frequency and hydrogen bonding angle suggests that it may be possible to disentangle electrostatic effects and effects of the orientation of hydrogen bonding on the nitrile stretching frequency. Meanwhile, the experimental measurement of the FTLS of the nitrile is an excellent tool to identify changes in the hydrogen bonding interactions for a particular probe.
中文翻译:

量化氢键对GFP中腈频率的影响:超越溶剂暴露
振动光谱是表征生物系统中复杂的非共价相互作用的有力工具。腈拉伸频率已被证明是一种特别方便的生物探针,但是由于其对局部氢键相互作用的敏感性,因此对腈光谱的解释变得复杂。通过要求了解探针周围局部环境的分子水平细节,这常常抑制了腈光谱的直接解释。尽管溶液中的小分子已充分证明了氢键对腈频率的影响,但在复杂的蛋白质系统中对这些作用的研究相对较少。为了解决这个问题,我们在整个绿色荧光蛋白(GFP)的9个位置引入了腈探针,并将每个探针的平均振动频率与分子动力学(MD)模拟中观察到的特定氢键几何形状进行了比较。我们表明,取决于每个腈的特定位置,发现了一个连续的氢键角,并且这些角度的差异解释了所测腈频率的差异。我们进一步观察到,即使在腈参与复杂且受限的氢键的情况下,腈频率的温度依赖性(以频率-温度线斜率,FTLS衡量)也很好地指示了探针的氢键相互作用。 ,无论是蛋白质还是水。虽然在解释所有腈键频率变化之前已对所有氢键环境施加了恒定的腈频率偏移量,但我们表明,在氢键可能受周围介质限制的系统中,这是不够的。然而,所观察到的腈频率和氢键角之间的相关性强度表明,可能可以消除静电效应和氢键取向对腈拉伸频率的影响。同时,腈FTLS的实验测量是识别特定探针氢键相互作用变化的出色工具。我们表明,在氢键可能受到周围介质限制的系统中,这是不够的。然而,所观察到的腈频率与氢键角之间的相关性强度表明,可能可以消除静电效应和氢键取向对腈拉伸频率的影响。同时,腈FTLS的实验测量是识别特定探针氢键相互作用变化的出色工具。我们表明,在氢键可能受到周围介质限制的系统中,这是不够的。然而,所观察到的腈频率和氢键角之间的相关性强度表明,可能可以消除静电效应和氢键取向对腈拉伸频率的影响。同时,腈FTLS的实验测量是识别特定探针氢键相互作用变化的出色工具。
更新日期:2018-06-27
中文翻译:

量化氢键对GFP中腈频率的影响:超越溶剂暴露
振动光谱是表征生物系统中复杂的非共价相互作用的有力工具。腈拉伸频率已被证明是一种特别方便的生物探针,但是由于其对局部氢键相互作用的敏感性,因此对腈光谱的解释变得复杂。通过要求了解探针周围局部环境的分子水平细节,这常常抑制了腈光谱的直接解释。尽管溶液中的小分子已充分证明了氢键对腈频率的影响,但在复杂的蛋白质系统中对这些作用的研究相对较少。为了解决这个问题,我们在整个绿色荧光蛋白(GFP)的9个位置引入了腈探针,并将每个探针的平均振动频率与分子动力学(MD)模拟中观察到的特定氢键几何形状进行了比较。我们表明,取决于每个腈的特定位置,发现了一个连续的氢键角,并且这些角度的差异解释了所测腈频率的差异。我们进一步观察到,即使在腈参与复杂且受限的氢键的情况下,腈频率的温度依赖性(以频率-温度线斜率,FTLS衡量)也很好地指示了探针的氢键相互作用。 ,无论是蛋白质还是水。虽然在解释所有腈键频率变化之前已对所有氢键环境施加了恒定的腈频率偏移量,但我们表明,在氢键可能受周围介质限制的系统中,这是不够的。然而,所观察到的腈频率和氢键角之间的相关性强度表明,可能可以消除静电效应和氢键取向对腈拉伸频率的影响。同时,腈FTLS的实验测量是识别特定探针氢键相互作用变化的出色工具。我们表明,在氢键可能受到周围介质限制的系统中,这是不够的。然而,所观察到的腈频率与氢键角之间的相关性强度表明,可能可以消除静电效应和氢键取向对腈拉伸频率的影响。同时,腈FTLS的实验测量是识别特定探针氢键相互作用变化的出色工具。我们表明,在氢键可能受到周围介质限制的系统中,这是不够的。然而,所观察到的腈频率和氢键角之间的相关性强度表明,可能可以消除静电效应和氢键取向对腈拉伸频率的影响。同时,腈FTLS的实验测量是识别特定探针氢键相互作用变化的出色工具。











































 京公网安备 11010802027423号
京公网安备 11010802027423号