Bioorganic Chemistry ( IF 4.5 ) Pub Date : 2018-06-06 , DOI: 10.1016/j.bioorg.2018.06.014 Wenjuan Zhang , Zhao Wei , Xueying Feng , Zhibing Zheng , Song Li
|
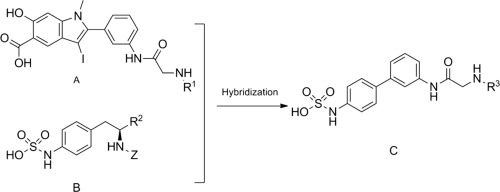
A series of novel (3′-amino-[1,1′-biphenyl]-4-yl) sulfamic acid derivatives were designed as nonphosphonate-based phosphotyrosy (pTyr) mimetics, synthesized and screened for use as HPTPβ inhibitors. Compounds C22 and C2 showed favorable HPTPβ inhibitory activity and better selectivity for HPTPβ than for PTP1B and SHP2. Docking results suggested that compounds C2 and C22 could not only efficiently fit into the catalytic site of the HPTPβ enzyme but also interact with the Lys1807, Arg1809 and Lys1811 residues of the secondary binding site, which was next to the catalytic center of the enzyme. The mode of interaction of the synthesized compound with the protein was different from the one found in a complex crystal of small molecules with HPTPβ (2I4H), in which the inhibitory molecule formed hydrogen bonds with the Gln1948 and Asn1735 residues of the secondary binding site.
中文翻译:

作为新型HPTPβ抑制剂的(3'-氨基-[1,1'-联苯] -4-基)氨基磺酸衍生物的合成及生物学评价
将一系列新颖的(3'-氨基-[1,1'-联苯] -4-基)氨基磺酸衍生物设计为基于非膦酸酯的磷酸酪氨酸(pTyr)模拟物,合成并筛选用作HPTPβ抑制剂。与PTP1B和SHP2相比,化合物C22和C2表现出良好的HPTPβ抑制活性和对HPTPβ的更好选择性。对接结果表明,化合物C2和C22不仅可以有效地插入HPTPβ酶的催化位点,而且还可以与次级结合位点的Lys1807,Arg1809和Lys1811残基相互作用,该残基紧挨着酶的催化中心。合成的化合物与蛋白质的相互作用方式与具有HPTPβ(2I4H)的小分子复杂晶体中发现的相互作用方式不同,











































 京公网安备 11010802027423号
京公网安备 11010802027423号