Acta Biomaterialia ( IF 9.4 ) Pub Date : 2018-06-06 , DOI: 10.1016/j.actbio.2018.06.012 Yun Wang , Feihu Wang , Ying Liu , Shaohui Xu , Yuanyuan Shen , Nianping Feng , Shengrong Guo
|
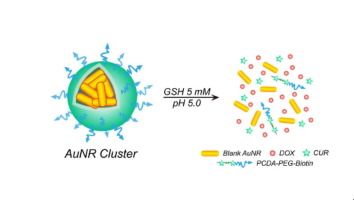
Effects of nanosized drug delivery systems on cancer are often compromised due to their low drug loadings, premature drug release and multi-drug resistance (MDR). Herein, we reported a glutathione detonated and pH responsive nano-cluster of Au nanorods (AuNRs) with chemotherapeutic doxorubicin (DOX) and pre-chemosensitizer polycurcumin to treat MCF-7/ADR cells. The nano-cluster was prepared by self-assembling of AuNRs conjugated with DOX and amphiphilic poly(curcumin-co-dithiodipropionic acid)-b-biotinylated poly(ethylene glycol) via an emulsion/solvent evaporation technique, termed AuNR Cluster. The AuNR Cluster had a high drug loading (31.5% DOX), presenting much better aqueous solubility and stability at physiological pH than their individual AuNRs. The AuNR Cluster could be detonated to be their individual AuNRs at an intracellular concentration level of glutathione (GSH) (5 mM) and triggered to release DOX at an acidic pH (pH 6.8 or 5.0), which effectively facilitated cellular uptake of DOX (607 vs 356 a.u. for AuNRs at 12 h) and inhibited DOX efflux (471.33 vs 39.17 a.u. for free DOX at 24 h). The IC50 value of DOX against MCF-7/ADR cells for AuNR Cluster was 4.15 µg/mL, much lower than that for free DOX (90.97 µg/mL). The AuNR Cluster took much more photothermal effects than their corresponding AuNRs and presented enhanced anti-tumor effect (IC50: 2.61 µg/mL) under 808 nm laser irradiation.
Statement of Significance
Nano-sized drug delivery systems for anti-MDR cancer is still a challenging task. Herein, AuNR Cluster is self-assembled by individual AuNRs via emulsion/solvent evaporation technique, having a structure consisting of hydrophobic DOX/PCDA-AuNR core and hydrophilic biotin-PEG chain shell. AuNR Cluster is detonated to disintegrate and yield its individual AuNRs at an intracellular concentration level of glutathione (5 mM) and triggered to release DOX at an acidic pH (6.8 or 5.0). In comparison with its individual AuNRs, AuNR Cluster has better water solubility and stability, greater photothermal effects under NIR irradiation, bigger cytotoxicity against MCF-7/ADR cells. AuNR Cluster is expected to be a potential nanomedicine for treatment of MDR cancer.
中文翻译:

谷胱甘肽引爆和pH响应的Au纳米棒与高剂量DOX的纳米簇,用于治疗多药耐药性癌症
纳米级药物输送系统对癌症的影响通常由于其低载药量,过早释放药物和多药耐药性(MDR)而受到影响。在本文中,我们报道了具有化学治疗性阿霉素(DOX)和化学增敏剂聚姜黄素的Au纳米棒(AuNRs)的谷胱甘肽引爆和pH响应纳米簇,用于治疗MCF-7 / ADR细胞。所述纳米团簇的制备是通过自组装与DOX和两亲性聚(姜黄素-共-二硫代二丙酸)缀合的AuNRs -b-生物素化的聚(乙二醇)经由乳液/溶剂蒸发技术,称为AuNR团簇。AuNR簇具有较高的药物载量(31.5%DOX),比其各自的AuNR具有更好的水溶性和在生理pH值下的稳定性。AuNR团簇可以在细胞内谷胱甘肽(GSH)(5 mM)浓度水平下引爆成它们各自的AuNRs,并触发在酸性pH(pH 6.8或5.0)下释放DOX,从而有效地促进了细胞对DOX的吸收(607)与12小时时AuNRs的356 au相比)和抑制了DOX外排(24小时时游离DOX的471.33 vs 39.17 au)。IC 50AuNR簇对MCF-7 / ADR细胞的DOX值为4.15 µg / mL,远低于游离DOX的90.97 µg / mL。在808 nm激光照射下,AuNR团簇比其相应的AuNRs具有更多的光热效应,并表现出增强的抗肿瘤效应(IC 50:2.61 µg / mL)。
重要声明
用于抗MDR癌症的纳米级药物输送系统仍然是一项艰巨的任务。在此,AuNR簇是通过乳液/溶剂蒸发技术由各个AuNRs自组装的,具有由疏水性DOX / PCDA-AuNR核心和亲水性生物素-PEG链壳组成的结构。引爆AuNR团簇以在细胞内浓度的谷胱甘肽(5 mM)分解并产生其单个AuNRs,并触发其在酸性pH(6.8或5.0)下释放DOX。与单个AuNRs相比,AuNR团簇具有更好的水溶性和稳定性,在近红外辐射下具有更好的光热效应,对MCF-7 / ADR细胞具有更大的细胞毒性。AuNR簇有望成为治疗MDR癌症的潜在纳米药物。











































 京公网安备 11010802027423号
京公网安备 11010802027423号