当前位置:
X-MOL 学术
›
ACS Infect. Dis.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Characterization of the Binding Sites for Bacterial Acyl Homoserine Lactones (AHLs) on Human Bitter Taste Receptors (T2Rs)
ACS Infectious Diseases ( IF 5.3 ) Pub Date : 2018-05-25 00:00:00 , DOI: 10.1021/acsinfecdis.8b00094 Appalaraju Jaggupilli 1 , Nisha Singh 1 , Vivianne Cruz De Jesus 1 , Kangmin Duan 1 , Prashen Chelikani 1
ACS Infectious Diseases ( IF 5.3 ) Pub Date : 2018-05-25 00:00:00 , DOI: 10.1021/acsinfecdis.8b00094 Appalaraju Jaggupilli 1 , Nisha Singh 1 , Vivianne Cruz De Jesus 1 , Kangmin Duan 1 , Prashen Chelikani 1
Affiliation
|
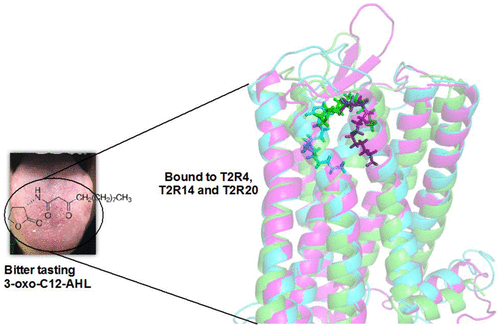
The 25 bitter taste receptors (T2Rs) in humans are novel players in mediating host–pathogen responses in the airways and innate immunity. The chemosensory T2Rs are expressed in different extraoral tissues and perform diverse pathophysiological roles from mediating bronchodilation to detecting bacterial infection in the airways. T2Rs were suggested to be activated by multiple bacterial quorum sensing molecules (QSMs). However, whether bacterial QSMs bind to T2Rs and the structural features on T2Rs has not yet been characterized. Here, we analyzed the taste sensory profiles of QSMs including acyl homoserine lactones (C4-AHL, C8-AHL, and 3-oxo-C12-AHL) and hydroxyquinolones (HHQ and NHQ) predominantly secreted by Gram-negative bacteria and characterized the candidate T2Rs interacting with different QSMs using structure–function approaches. The potency of the above QSMs for T2Rs significantly expressed in the airways, namely T2R4, T2R14, and T2R20, was characterized. 3-Oxo-C12-AHL activated T2R4, T2R14, and T2R20, while C8-AHL activated T2R4 and T2R14 with strong potency. The T2R amino acid residues involved in the interactions were characterized by molecular-model-guided site-directed mutagenesis. AHLs bind to a similar orthosteric site present on the extracellular surface in all three T2Rs with significant contributions from residues in extracellular loop 2. Our results reveal the mode of binding of AHLs for different T2Rs and provide biochemical insights into their interactions. This study will facilitate mechanistic studies aimed at understanding the role of these T2Rs as “sensors” of bacteria and in host–pathogen interactions.
中文翻译:

人体苦味受体(T2R)上的细菌酰基高丝氨酸内酯(AHL)的结合位点的表征
人类中的25种苦味觉受体(T2Rs)是介导气道中宿主-病原体反应和先天免疫的新型参与者。化学感觉T2Rs在不同的口腔外组织中表达,并发挥多种病理生理作用,从介导支气管扩张到检测气道细菌感染。T2Rs被多种细菌群体感应分子(QSM)激活。但是,细菌QSMs是否与T2Rs结合以及T2Rs上的结构特征尚未得到表征。在这里,我们分析了主要由革兰氏阴性细菌分泌的QSM的味觉特征,包括酰基高丝氨酸内酯(C4-AHL,C8-AHL和3-oxo-C12-AHL)和羟基喹诺酮(HHQ和NHQ)。 T2R使用结构-功能方法与不同的QSM进行交互。表征了以上QSM对在气道中显着表达的T2R的效力,即T2R4,T2R14和T2R20。3-Oxo-C12-AHL激活T2R4,T2R14和T2R20,而C8-AHL激活T2R4和T2R14具有很强的效力。参与相互作用的T2R氨基酸残基的特征在于分子模型指导的定点诱变。AHL与所有三个T2R中存在于细胞外表面的类似正构位点结合,这是细胞外环2中残基的重要贡献。我们的结果揭示了AHL与不同T2R结合的方式,并为其相互作用提供了生化方面的见识。这项研究将有助于旨在了解这些T2R作为细菌“传感器”以及在宿主与病原体相互作用中的作用的机理研究。
更新日期:2018-05-25
中文翻译:

人体苦味受体(T2R)上的细菌酰基高丝氨酸内酯(AHL)的结合位点的表征
人类中的25种苦味觉受体(T2Rs)是介导气道中宿主-病原体反应和先天免疫的新型参与者。化学感觉T2Rs在不同的口腔外组织中表达,并发挥多种病理生理作用,从介导支气管扩张到检测气道细菌感染。T2Rs被多种细菌群体感应分子(QSM)激活。但是,细菌QSMs是否与T2Rs结合以及T2Rs上的结构特征尚未得到表征。在这里,我们分析了主要由革兰氏阴性细菌分泌的QSM的味觉特征,包括酰基高丝氨酸内酯(C4-AHL,C8-AHL和3-oxo-C12-AHL)和羟基喹诺酮(HHQ和NHQ)。 T2R使用结构-功能方法与不同的QSM进行交互。表征了以上QSM对在气道中显着表达的T2R的效力,即T2R4,T2R14和T2R20。3-Oxo-C12-AHL激活T2R4,T2R14和T2R20,而C8-AHL激活T2R4和T2R14具有很强的效力。参与相互作用的T2R氨基酸残基的特征在于分子模型指导的定点诱变。AHL与所有三个T2R中存在于细胞外表面的类似正构位点结合,这是细胞外环2中残基的重要贡献。我们的结果揭示了AHL与不同T2R结合的方式,并为其相互作用提供了生化方面的见识。这项研究将有助于旨在了解这些T2R作为细菌“传感器”以及在宿主与病原体相互作用中的作用的机理研究。



























 京公网安备 11010802027423号
京公网安备 11010802027423号