当前位置:
X-MOL 学术
›
Angew. Chem. Int. Ed.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Functionalizable Stereocontrolled Cyclopolyethers by Ring‐Closing Metathesis as Natural Polymer Mimics
Angewandte Chemie International Edition ( IF 16.1 ) Pub Date : 2018-06-20 , DOI: 10.1002/anie.201805113 Mohammed Alkattan 1, 2 , Joëlle Prunet 2 , Michael P. Shaver 1
Angewandte Chemie International Edition ( IF 16.1 ) Pub Date : 2018-06-20 , DOI: 10.1002/anie.201805113 Mohammed Alkattan 1, 2 , Joëlle Prunet 2 , Michael P. Shaver 1
Affiliation
|
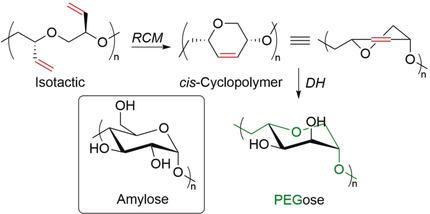
Whereas complex stereoregular cyclic architectures are commonplace in biomacromolecules, they remain rare in synthetic polymer chemistry, thus limiting the potential to develop synthetic mimics or advanced materials for biomedical applications. Herein we disclose the formation of a stereocontrolled 1,4‐linked six‐membered cyclopolyether prepared by ring‐closing metathesis (RCM). Ru‐mediated RCM, with careful control of the catalyst, concentration, and temperature, selectively affords the six‐membered‐ring cyclopolymer. Under optimized reaction conditions, no metathetical degradation, macrocycle formation, or cross‐linking was observed. Post‐polymerization modification by dihydroxylation afforded a novel polymer family encompassing a poly(ethylene glycol) backbone and sugar‐like functionalities (“PEGose”). This strategy also paves the way for using RCM as an efficient method to synthesize other stereocontrolled cyclopolymers.
中文翻译:

通过闭环复分解作为天然聚合物模拟物可官能化的立体控制环聚醚。
尽管复杂的立体规则环状结构在生物大分子中很常见,但在合成高分子化学中仍然很少见,因此限制了开发用于生物医学应用的合成模拟物或先进材料的潜力。本文中,我们公开了通过闭环复分解(RCM)制备的立体控制的1,4连接的六元环聚醚的形成。Ru介导的RCM,在仔细控制催化剂,浓度和温度的情况下,有选择地提供了六元环的环状聚合物。在优化的反应条件下,未观察到易位降解,大环形成或交联。通过二羟基化作用进行的聚合后修饰提供了一个新颖的聚合物家族,其中包括聚乙二醇主链和类似糖的官能团(“ PEGose”)。
更新日期:2018-06-20
中文翻译:

通过闭环复分解作为天然聚合物模拟物可官能化的立体控制环聚醚。
尽管复杂的立体规则环状结构在生物大分子中很常见,但在合成高分子化学中仍然很少见,因此限制了开发用于生物医学应用的合成模拟物或先进材料的潜力。本文中,我们公开了通过闭环复分解(RCM)制备的立体控制的1,4连接的六元环聚醚的形成。Ru介导的RCM,在仔细控制催化剂,浓度和温度的情况下,有选择地提供了六元环的环状聚合物。在优化的反应条件下,未观察到易位降解,大环形成或交联。通过二羟基化作用进行的聚合后修饰提供了一个新颖的聚合物家族,其中包括聚乙二醇主链和类似糖的官能团(“ PEGose”)。











































 京公网安备 11010802027423号
京公网安备 11010802027423号