当前位置:
X-MOL 学术
›
ACS Cent. Sci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Surface Pore Engineering of Covalent Organic Frameworks for Ammonia Capture through Synergistic Multivariate and Open Metal Site Approaches
ACS Central Science ( IF 12.7 ) Pub Date : 2018-06-06 00:00:00 , DOI: 10.1021/acscentsci.8b00232 Yajie Yang 1 , Muhammad Faheem 1 , Lili Wang 1 , Qinghao Meng 1 , Haoyan Sha 2 , Nan Yang 3 , Ye Yuan 1 , Guangshan Zhu 1
ACS Central Science ( IF 12.7 ) Pub Date : 2018-06-06 00:00:00 , DOI: 10.1021/acscentsci.8b00232 Yajie Yang 1 , Muhammad Faheem 1 , Lili Wang 1 , Qinghao Meng 1 , Haoyan Sha 2 , Nan Yang 3 , Ye Yuan 1 , Guangshan Zhu 1
Affiliation
|
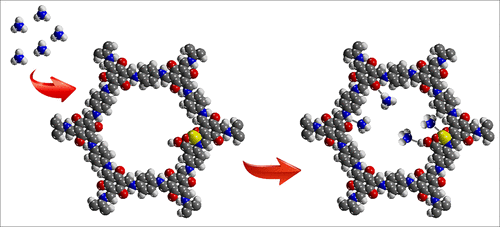
Ammonia (NH3) is a commonly used industrial gas, but its corrosiveness and toxicity are hazardous to human health. Although many adsorbents have been investigated for NH3 sorption, limited ammonia uptake remains an urgent issue yet to be solved. In this article, a series of multivariate covalent organic frameworks (COFs) are explored which are densely functionalized with various active groups, such as —N—H, —C═O, and carboxyl group. Then, a metal ion (Ca2+, Mn2+, and Sr2+) is integrated into the carboxylated structure achieving the first case of an open metal site in COF architecture. X-ray photoelectron spectroscopy reveals conclusive evidence for the multiple binding interactions with ammonia in the modified COF materials. Infrared spectroscopy indicates a general trend of binding capability from weak to strong along with —N—H, —C═O, carboxyl group, and metal ion. Through the synergistic multivariate and open metal site, the COF materials show excellent adsorption capacities (14.3 and 19.8 mmol g–1 at 298 and 283 K, respectively) and isosteric heat (Qst) of 91.2 kJ mol–1 for ammonia molecules. This novel approach enables the development of tailor-made porous materials with tunable pore-engineered surface for ammonia uptake.
中文翻译:

通过协同多元和开放金属位点方法捕获氨的共价有机框架的表面孔隙工程
氨气(NH 3)是一种常用的工业气体,但其腐蚀性和毒性对人体健康有害。尽管已经研究了许多吸附剂对NH 3的吸附,但是氨吸收受限仍然是一个亟待解决的紧迫问题。在本文中,研究了一系列多元共价有机骨架(COF),这些骨架被各种活性基团(例如-NH,-C = O和羧基)密集地官能化。然后,金属离子(Ca 2 +,Mn 2+和Sr 2+)被整合到羧化结构中,实现了COF体系结构中开放金属位点的第一种情况。X射线光电子能谱揭示了改性COF材料中与氨的多重结合相互作用的确凿证据。红外光谱表明结合能力的总体趋势是从弱到强以及-NH,-C = O,羧基和金属离子。通过多变量协同作用和开放金属位点,COF材料显示出优异的吸附能力(分别在298 K和283 K时分别为14.3和19.8 mmol g –1)和等规热(Q st)为91.2 kJ mol –1用于氨分子。这种新颖的方法使得能够开发具有可调节的孔工程表面以吸收氨的量身定制的多孔材料。
更新日期:2018-06-06
中文翻译:

通过协同多元和开放金属位点方法捕获氨的共价有机框架的表面孔隙工程
氨气(NH 3)是一种常用的工业气体,但其腐蚀性和毒性对人体健康有害。尽管已经研究了许多吸附剂对NH 3的吸附,但是氨吸收受限仍然是一个亟待解决的紧迫问题。在本文中,研究了一系列多元共价有机骨架(COF),这些骨架被各种活性基团(例如-NH,-C = O和羧基)密集地官能化。然后,金属离子(Ca 2 +,Mn 2+和Sr 2+)被整合到羧化结构中,实现了COF体系结构中开放金属位点的第一种情况。X射线光电子能谱揭示了改性COF材料中与氨的多重结合相互作用的确凿证据。红外光谱表明结合能力的总体趋势是从弱到强以及-NH,-C = O,羧基和金属离子。通过多变量协同作用和开放金属位点,COF材料显示出优异的吸附能力(分别在298 K和283 K时分别为14.3和19.8 mmol g –1)和等规热(Q st)为91.2 kJ mol –1用于氨分子。这种新颖的方法使得能够开发具有可调节的孔工程表面以吸收氨的量身定制的多孔材料。










































 京公网安备 11010802027423号
京公网安备 11010802027423号