Molecular Catalysis ( IF 4.6 ) Pub Date : 2018-06-05 , DOI: 10.1016/j.mcat.2018.05.029 Dongsong Zheng , Rui Liu , Yu Wang , Tanyu Cheng , Guohua Liu
|
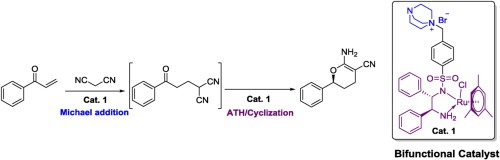
Tandem catalytic reaction in one-pot possesses remarkable advantages and is a powerful methodology for the synthesis of high value chemicals. Asymmetric transfer hydrogenation catalyzed by Ru-TsDPEN type catalysts, developed Noyori and Ikariya, is a useful strategy for constructing chiral alcohols and amines. Herein, a novel bifunctional Noyori-Ikariya type catalyst was developed, which displays good catalytic efficiency for Michael addition/ATH/cyclization tandem reaction. A series of chiral 3,4-dihydro-2H-pyran-5-carbonitriles were synthesized in good yield (up to 79%) and with excellent enantioselectivity (up to 97%) via this one-pot reaction under mild conditions.
中文翻译:

通过双功能Noyori-Ikariya型催化剂催化的Michael串联/迈克尔加成/不对称转移氢化/环化反应,高度手性地合成手性3,4-二氢-2 H-吡喃-5-腈
一锅式串联催化反应具有显着优势,是合成高价值化学品的有力方法。由Noyori和Ikariya开发的Ru-TsDPEN型催化剂催化的不对称转移氢化是构建手性醇和胺的有用策略。在此,开发了一种新型的双官能Noyori-Ikariya型催化剂,其对迈克尔加成/ ATH /环化串联反应显示出良好的催化效率。通过在温和条件下的一锅反应,合成了一系列手性3,4-二氢-2 H-吡喃-5-腈,收率高(高达79%),对映选择性(高达97%)。




























 京公网安备 11010802027423号
京公网安备 11010802027423号