当前位置:
X-MOL 学术
›
J. Phys. Chem. A
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Influence of Microheterogeneous Environments of Sodium Dodecyl Sulfate on the Kinetics of Oxidation of l-Serine by Chloro and Chlorohydroxo Complexes of Gold(III)
The Journal of Physical Chemistry A ( IF 2.7 ) Pub Date : 2018-06-06 00:00:00 , DOI: 10.1021/acs.jpca.8b02409 Krishnendu Maiti 1 , Pratik K. Sen 1 , Anil K. Barik 2 , Biswajit Pal 2
The Journal of Physical Chemistry A ( IF 2.7 ) Pub Date : 2018-06-06 00:00:00 , DOI: 10.1021/acs.jpca.8b02409 Krishnendu Maiti 1 , Pratik K. Sen 1 , Anil K. Barik 2 , Biswajit Pal 2
Affiliation
|
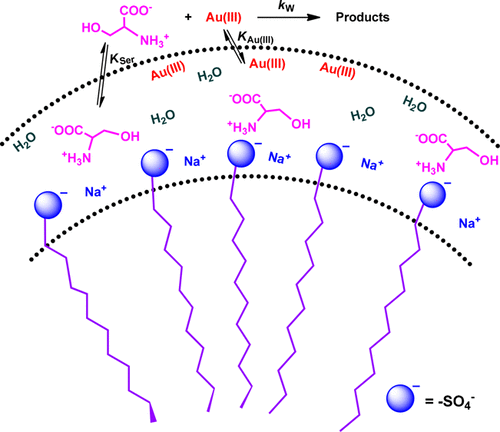
The oxidation of l-serine by chloro and chlorohydroxo complexes of gold(III) was spectrophotometrically investigated in acidic buffer media in the absence and presence of the anionic surfactant sodium dodecyl sulfate (SDS). The oxidation rate decreases with increase in either [H+] or [Cl–]. Gold(III) complex species react with the zwitterionic form of serine to yield acetaldehyde (principal reaction product) through oxidative decarboxylation and subsequent deamination processes. A reaction pathway involving one electron transfer from serine to Au(III) followed by homolytic cleavage of α-C–C bond with the concomitant formation of iminic cation intermediate has been proposed where Au(III) is initially reduced to Au(II). The surfactant in the submicellar region exhibits a catalytic effect on the reaction rate at [SDS] ≤ 4 mM; however, in the postmicellar region an inhibitory effect was prominent at [SDS] ≥ 4 mM. The catalytic effect below the critical micelle concentration (cmc) may be attributable to the electrostatic attraction between serine and SDS that, in turn, enhances the nucleophilicity of the carboxylate ion of the amino acid. The inhibition effect beyond cmc has been explained by considering the distribution of the reactant species between the aqueous and the micellar pseudophases that restricts the close association of the reactant species. The thermodynamic parameters ΔH0 and ΔS0 associated with the binding between serine and SDS micelle were calculated to be −14.4 ± 2 kJ mol–1 and −6.3 ± 0.5 J K–1 mol–1, respectively. Water structure rearrangement and micelle–substrate binding play instrumental roles during the transfer of the reactant species from aqueous to micellar pseudophase.
中文翻译:

十二烷基硫酸钠微多相环境对金(III)的氯和氯羟基络合物氧化l-丝氨酸动力学的影响
在不存在和存在阴离子表面活性剂十二烷基硫酸钠(SDS)的情况下,在酸性缓冲液中分光光度法研究了金(Ⅲ)的氯和氯羟基络合物对1-丝氨酸的氧化作用。氧化速率随[H + ]或[Cl –]。金(III)配合物物种与丝氨酸的两性离子形式反应,通过氧化脱羧和随后的脱氨过程生成乙醛(主要反应产物)。已经提出了一种反应途径,该途径涉及一个电子从丝氨酸转移到Au(III),然后均相裂解α-CC键并伴随亚胺阳离子中间体的形成,其中Au(III)最初被还原为Au(II)。在[SDS]≤4 mM时,在胶束下区域的表面活性剂显示出对反应速率的催化作用。但是,在胶束后区域,[SDS]≥4 mM时抑制作用显着。低于临界胶束浓度(cmc)的催化作用可能归因于丝氨酸和SDS之间的静电吸引,进而,增强氨基酸的羧酸根离子的亲核性。通过考虑反应物种类在水相和胶束假相之间的分布来解释超出cmc的抑制作用,该分布限制了反应物种类的紧密缔合。热力学参数Δ与丝氨酸和SDS胶束之间的结合相关的H 0和ΔS 0分别计算为-14.4±2 kJ mol –1和-6.3±0.5 JK –1 mol –1。在反应物从水相到胶束假相的转移过程中,水的结构重排和胶束-底物的结合起着重要的作用。
更新日期:2018-06-06
中文翻译:

十二烷基硫酸钠微多相环境对金(III)的氯和氯羟基络合物氧化l-丝氨酸动力学的影响
在不存在和存在阴离子表面活性剂十二烷基硫酸钠(SDS)的情况下,在酸性缓冲液中分光光度法研究了金(Ⅲ)的氯和氯羟基络合物对1-丝氨酸的氧化作用。氧化速率随[H + ]或[Cl –]。金(III)配合物物种与丝氨酸的两性离子形式反应,通过氧化脱羧和随后的脱氨过程生成乙醛(主要反应产物)。已经提出了一种反应途径,该途径涉及一个电子从丝氨酸转移到Au(III),然后均相裂解α-CC键并伴随亚胺阳离子中间体的形成,其中Au(III)最初被还原为Au(II)。在[SDS]≤4 mM时,在胶束下区域的表面活性剂显示出对反应速率的催化作用。但是,在胶束后区域,[SDS]≥4 mM时抑制作用显着。低于临界胶束浓度(cmc)的催化作用可能归因于丝氨酸和SDS之间的静电吸引,进而,增强氨基酸的羧酸根离子的亲核性。通过考虑反应物种类在水相和胶束假相之间的分布来解释超出cmc的抑制作用,该分布限制了反应物种类的紧密缔合。热力学参数Δ与丝氨酸和SDS胶束之间的结合相关的H 0和ΔS 0分别计算为-14.4±2 kJ mol –1和-6.3±0.5 JK –1 mol –1。在反应物从水相到胶束假相的转移过程中,水的结构重排和胶束-底物的结合起着重要的作用。











































 京公网安备 11010802027423号
京公网安备 11010802027423号