Acta Biomaterialia ( IF 9.4 ) Pub Date : 2018-06-05 , DOI: 10.1016/j.actbio.2018.06.004 Joao Paulo Zambon , In Kap Ko , Mehran Abolbashari , Jennifer Huling , Cara Clouse , Tae Hyoung Kim , Charesa Smith , Anthony Atala , James J. Yoo
|
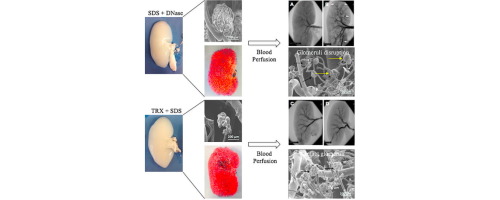
Kidney transplantation is currently the only definitive solution for the treatment of end-stage renal disease (ESRD), however transplantation is severely limited by the shortage of available donor kidneys. Recent progress in whole organ engineering based on decellularization/recellularization techniques has enabled pre-clinical in vivo studies using small animal models; however, these in vivo studies have been limited to short-term assessments. We previously developed a decellularization system that effectively removes cellular components from porcine kidneys. While functional re-endothelialization on the porcine whole kidney scaffold was able to improve vascular patency, as compared to the kidney scaffold only, the duration of patency lasted only a few hours. In this study, we hypothesized that significant damage in the microvasculatures within the kidney scaffold resulted in the cessation of blood flow, and that thorough investigation is necessary to accurately evaluate the vascular integrity of the kidney scaffolds. Two decellularization protocols [sodium dodecyl sulfate (SDS) with DNase (SDS+DNase) or Triton X-100 with SDS (TRX+SDS)] were used to evaluate and optimize the levels of vascular integrity within the kidney scaffold. Results from vascular analysis studies using vascular corrosion casting and angiograms demonstrated that the TRX+SDS method was able to better maintain intact and functional microvascular architectures such as glomeruli within the acellular matrices than that by the SDS+DNase treatment. Importantly, in vitro blood perfusion of the re-endothelialized kidney construct revealed improved vascular function of the scaffold by TRX+SDS treatment compared with the SDS+DNase. Our results suggest that the optimized TRX+SDS decellularization method preserves kidney-specific microvasculatures and may contribute to long-term vascular patency following implantation.
Statement of Significance
Kidney transplantation is the only curative therapy for patients with end-stage renal disease (ESRD). However, in the United States, the supply of donor kidneys meets less than one-fifth of the demand; and those patients that receive a donor kidney need life-long immunosuppressive therapy to avoid organ rejection. In the last two decades, regenerative medicine and tissue engineering have emerged as an attractive alternative to overcome these limitations.
In 2013, Song et al published the first experimental orthotopic transplantation of a bioengineering kidney in rodents. In this study, they demonstrated evidences of kidney tissue regeneration and partial function restoration. Despite these initial promising results, there are still many challenges to achieve long-term blood perfusion without graft thrombosis. In this paper, we demonstrated that perfusion of detergents through the renal artery of porcine kidneys damages the glomeruli microarchitecture as well as peritubular capillaries. Modifying dynamic parameters such as flow rate, detergent concentration, and decellularization time, we were able to establish an optimized decellularization protocol with no evidences of disruption of glomeruli microarchitecture. As a proof of concept, we recellularized the kidney scaffolds with endothelial cells and in vitro perfused whole porcine blood successfully for 24h with no evidences of thrombosis.
中文翻译:

两种维持功能性血管结构的猪肾脏脱细胞方法的比较分析
肾脏移植目前是治疗终末期肾脏疾病(ESRD)的唯一确定性解决方案,但是由于缺乏可用的供体肾脏,移植受到了严重限制。基于脱细胞/再细胞化技术的全器官工程学的最新进展使得能够使用小动物模型进行临床前体内研究。但是,这些体内研究仅限于短期评估。我们之前开发了一种脱细胞系统,该系统可有效去除猪肾脏中的细胞成分。尽管与仅肾支架相比,猪全肾支架上的功能性重新内皮化能够改善血管通畅性,但通畅的持续时间仅持续数小时。在这项研究中,我们假设肾脏支架内的微血管严重受损导致血流停止,因此有必要进行全面研究以准确评估肾脏支架的血管完整性。两种脱细胞方案[带有DNase的十二烷基硫酸钠(SDS)(SDS + DNase)或带有SDS的Triton X-100(TRX + SDS)]用于评估和优化肾脏支架内的血管完整性水平。使用血管腐蚀铸模和血管造影照片进行的血管分析研究结果表明,与SDS + DNase处理相比,TRX + SDS方法能够更好地保持无细胞基质内完整和功能性的微血管结构,例如肾小球。重要的,与SDS + DNase相比,经重新血管内皮化的肾脏构建体的体外血液灌注显示,TRX + SDS处理可改善支架的血管功能。我们的结果表明,优化的TRX + SDS脱细胞方法可保留肾脏特有的微脉管系统,并可能有助于植入后的长期血管通畅。
重要声明
肾脏移植是终末期肾病(ESRD)患者的唯一治疗方法。但是,在美国,供体肾脏的供应量不足需求量的五分之一。那些接受供体肾脏的患者需要终生免疫抑制治疗,以避免器官排斥。在过去的二十年中,再生医学和组织工程学已经成为克服这些局限性的有吸引力的替代方法。
2013年,Song等人在啮齿动物中首次发表了生物工程肾的实验性原位移植。在这项研究中,他们证明了肾脏组织再生和部分功能恢复的证据。尽管取得了这些最初令人鼓舞的结果,但要实现长期的血液灌输而不移植血栓形成仍然存在许多挑战。在本文中,我们证明了通过猪肾脏的肾动脉灌注去污剂会损害肾小球的微结构以及肾小管周围的毛细血管。修改动态参数,例如流速,去污剂浓度和脱细胞时间,我们能够建立优化的脱细胞方案,而没有丝毫肾小球微结构破坏的迹象。作为概念证明,猪全血在体外成功灌注24h,没有血栓形成的迹象。







































 京公网安备 11010802027423号
京公网安备 11010802027423号