Bioorganic Chemistry ( IF 4.5 ) Pub Date : 2018-06-05 , DOI: 10.1016/j.bioorg.2018.06.007 Bilquees Bano , Kanwal , Khalid Mohammed Khan , Arif Lodhi , Uzma Salar , Farida Begum , Muhammad Ali , Muhammad Taha , Shahnaz Perveen
|
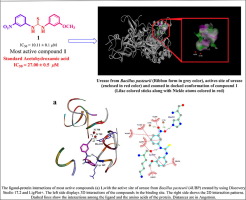
The current study deals with the synthesis of urea and thiourea derivatives 1–37 which were characterized by various spectroscopic techniques including FAB-MS, 1H-, and 13C NMR. The synthetic compounds were subjected to urease inhibitory activity and compounds exhibited good to moderate urease inhibitory activity having IC50 values in range of 10.11–69.80 µM. Compound 1 (IC50 = 10.11 ± 0.11 µM) was found to be most active and even better as compared to the standard acetohydroxamic acid (IC50 = 27.0 ± 0.5 µM). A limited structure–activity relationship (SAR) was established and the compounds were also subjected to docking studies to confirm the binding interactions of ligands (compounds) with the active site of enzyme.
中文翻译:

硫脲和尿素衍生物的合成,体外脲酶抑制活性以及分子对接研究
与尿素的合成目前的研究优惠和硫脲衍生物1 - 37,其进行了表征通过各种光谱技术包括FAB-MS,1 H-和13 C NMR。合成的化合物具有脲酶抑制活性,化合物表现出良好到中等的脲酶抑制活性,IC 50值为10.11–69.80 µM。 与标准乙酰氧肟酸(IC 50)相比,化合物1(IC 50 = 10.11±0.11 µM)具有最高的活性,甚至更好。 = 27.0±0.5 µM)。建立了有限的结构-活性关系(SAR),并对化合物也进行了对接研究,以确认配体(化合物)与酶的活性位点之间的结合相互作用。











































 京公网安备 11010802027423号
京公网安备 11010802027423号