Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Rattle-Structured Rough Nanocapsules with in-Situ-Formed Gold Nanorod Cores for Complementary Gene/Chemo/Photothermal Therapy
ACS Nano ( IF 17.1 ) Pub Date : 2018-06-05 00:00:00 , DOI: 10.1021/acsnano.8b01440 Xinyan Chen 1, 2, 3 , Qing Zhang 1, 2, 3 , Jinliang Li 4 , Ming Yang 4 , Nana Zhao 1, 2, 3 , Fu-Jian Xu 1, 2, 3
ACS Nano ( IF 17.1 ) Pub Date : 2018-06-05 00:00:00 , DOI: 10.1021/acsnano.8b01440 Xinyan Chen 1, 2, 3 , Qing Zhang 1, 2, 3 , Jinliang Li 4 , Ming Yang 4 , Nana Zhao 1, 2, 3 , Fu-Jian Xu 1, 2, 3
Affiliation
|
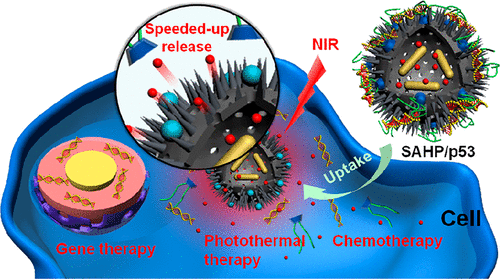
The morphology of nanoparticles influences their cellular uptake process, while rough surface-enhanced affinity renders rough nanoparticles desirable in related biomedical applications. In this work, rattle-structured rough nanocapsules ([email protected], AHPs) composed of in-situ-formed gold nanorod (Au NR) cores and polycationic mesoporous silica shells were constructed for trimodal complementary cancer therapy. Taking advantage of surface roughness, near-infrared (NIR) responsiveness, and controlled release manner, AHPs were expected to realize the co-delivery of sorafenib (SF, a hydrophobic antiproliferative and antiangiogenic drug) and antioncogene p53 for malignant hepatocellular carcinoma treatment. The rough surface feature of AHP was investigated for cellular uptake and the subsequent gene transfection. The feasibility of photothermal Au NR cores for NIR-triggered SF release was also tested. Notably, synergistic effects based on photothemal therapy-enhanced chemotherapy were achieved. In addition, the good in vivo performance of the proposed multifunctional nanoparticles with rough surfaces was also demonstrated. The current work extends the biomedical applications of the intriguing rough nanoparticles and provides a facile strategy to construct flexible platforms for complementary gene/chemo/photothermal therapy.
中文翻译:

具有互补基因/化学/光热疗法原位形成的金纳米棒核的网状结构的粗糙纳米胶囊。
纳米颗粒的形态影响其细胞摄取过程,而粗糙的表面增强的亲和力使粗糙的纳米颗粒成为相关生物医学应用中所需要的。在这项工作中,由原位组成的拨浪鼓结构的粗糙纳米胶囊([电子邮件保护],AHP)形成金纳米棒(Au NR)核和聚阳离子介孔二氧化硅壳用于三峰互补癌症治疗。利用表面粗糙度,近红外(NIR)反应性和控释方式,AHP有望实现索拉非尼(SF,一种疏水性抗增殖和抗血管生成药物)和抗癌基因p53共同递送用于恶性肝细胞癌的治疗。研究了AHP的粗糙表面特征,以用于细胞吸收和随后的基因转染。还测试了光热Au NR核用于NIR触发的SF释放的可行性。值得注意的是,获得了基于光热疗法增强化学疗法的协同作用。另外,体内好还证明了所提出的具有粗糙表面的多功能纳米颗粒的性能。当前的工作扩展了有趣的粗糙纳米颗粒的生物医学应用,并提供了一种简便的策略来构建用于互补基因/化学/光热疗法的灵活平台。
更新日期:2018-06-05
中文翻译:

具有互补基因/化学/光热疗法原位形成的金纳米棒核的网状结构的粗糙纳米胶囊。
纳米颗粒的形态影响其细胞摄取过程,而粗糙的表面增强的亲和力使粗糙的纳米颗粒成为相关生物医学应用中所需要的。在这项工作中,由原位组成的拨浪鼓结构的粗糙纳米胶囊([电子邮件保护],AHP)形成金纳米棒(Au NR)核和聚阳离子介孔二氧化硅壳用于三峰互补癌症治疗。利用表面粗糙度,近红外(NIR)反应性和控释方式,AHP有望实现索拉非尼(SF,一种疏水性抗增殖和抗血管生成药物)和抗癌基因p53共同递送用于恶性肝细胞癌的治疗。研究了AHP的粗糙表面特征,以用于细胞吸收和随后的基因转染。还测试了光热Au NR核用于NIR触发的SF释放的可行性。值得注意的是,获得了基于光热疗法增强化学疗法的协同作用。另外,体内好还证明了所提出的具有粗糙表面的多功能纳米颗粒的性能。当前的工作扩展了有趣的粗糙纳米颗粒的生物医学应用,并提供了一种简便的策略来构建用于互补基因/化学/光热疗法的灵活平台。



























 京公网安备 11010802027423号
京公网安备 11010802027423号