当前位置:
X-MOL 学术
›
Biochemistry
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
The “Recognition Helix” of the Type II Acyl Carrier Protein (ACP) Utilizes a “Ubiquitin Interacting Motif (UIM)”-like Surface To Bind Its Partners
Biochemistry ( IF 2.9 ) Pub Date : 2018-06-05 00:00:00 , DOI: 10.1021/acs.biochem.8b00220 Usha Yadav 1 , Richa Arya 2 , Suman Kundu 2 , Monica Sundd 1
Biochemistry ( IF 2.9 ) Pub Date : 2018-06-05 00:00:00 , DOI: 10.1021/acs.biochem.8b00220 Usha Yadav 1 , Richa Arya 2 , Suman Kundu 2 , Monica Sundd 1
Affiliation
|
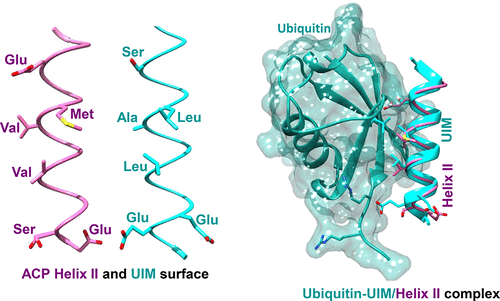
Interaction interfaces comprise a finite number of sequence/structural motifs, which are often repeated in nature. Here we show how a helical motif present in the acyl carrier protein (ACP), involved in multiprotein interactions, displays a binding interface similar to that of the “ubiquitin interacting motif (UIM)”. Analysis of the crystal structures of the ACP–enzyme complexes gave the first hint that helix II of the ACP (“universal recognition helix”) utilizes UIM-like noncovalent interactions to associate with the type II fatty acid biosynthesis (FAS) pathway enzymes. The ACP interacting functional surface of the FAS enzymes comprises positively charged residues, flanking a central hydrophobic patch, akin to ubiquitin. Our nuclear magnetic resonance chemical shift perturbation studies, relaxation studies, and surface plasmon resonance measurements unequivocally show that helix II of ACP behaves like a UIM motif in its interactions with ubiquitin, by binding the Ile 44 functional surface of the latter. A synthetic peptide with the ACP helix II sequence showed equivalent binding to ubiquitin. The evolution of similar interaction motifs in the two systems has probably been driven by functional constraints, as both ACP and UIM participate in multiprotein interactions.
中文翻译:

II型酰基载体蛋白(ACP)的“识别螺旋”利用类似“泛素相互作用基序(UIM)”的表面结合其伙伴
交互界面包括有限数量的序列/结构基序,这些序列/结构基序在自然界中经常被重复。在这里,我们显示了存在于多蛋白相互作用中的酰基载体蛋白(ACP)中存在的螺旋基序如何显示与“泛素相互作用基序(UIM)”相似的结合界面。对ACP-酶复合物晶体结构的分析首次提示ACP的螺旋II(“通用识别螺旋”)利用UIM样非共价相互作用与II型脂肪酸生物合成(FAS)途径酶相关联。FAS酶的ACP相互作用功能表面包含带正电荷的残基,侧翼是类似于泛素的中央疏水性补丁。我们的核磁共振化学位移扰动研究,弛豫研究,表面等离振子共振测量清楚地表明,ACP的螺旋II通过与泛素的Ile 44功能表面结合,在与泛素的相互作用中表现得像UIM模体。具有ACP螺旋II序列的合成肽显示出与泛素的等效结合。由于ACP和UIM都参与了多蛋白相互作用,因此两个系统中相似相互作用基序的进化可能是由功能限制所驱动的。
更新日期:2018-06-05
中文翻译:

II型酰基载体蛋白(ACP)的“识别螺旋”利用类似“泛素相互作用基序(UIM)”的表面结合其伙伴
交互界面包括有限数量的序列/结构基序,这些序列/结构基序在自然界中经常被重复。在这里,我们显示了存在于多蛋白相互作用中的酰基载体蛋白(ACP)中存在的螺旋基序如何显示与“泛素相互作用基序(UIM)”相似的结合界面。对ACP-酶复合物晶体结构的分析首次提示ACP的螺旋II(“通用识别螺旋”)利用UIM样非共价相互作用与II型脂肪酸生物合成(FAS)途径酶相关联。FAS酶的ACP相互作用功能表面包含带正电荷的残基,侧翼是类似于泛素的中央疏水性补丁。我们的核磁共振化学位移扰动研究,弛豫研究,表面等离振子共振测量清楚地表明,ACP的螺旋II通过与泛素的Ile 44功能表面结合,在与泛素的相互作用中表现得像UIM模体。具有ACP螺旋II序列的合成肽显示出与泛素的等效结合。由于ACP和UIM都参与了多蛋白相互作用,因此两个系统中相似相互作用基序的进化可能是由功能限制所驱动的。











































 京公网安备 11010802027423号
京公网安备 11010802027423号