当前位置:
X-MOL 学术
›
Green Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Imidazolium-based dicationic ionic liquids: highly efficient extractants for separating aromatics from aliphatics
Green Chemistry ( IF 9.8 ) Pub Date : 2018-06-05 , DOI: 10.1039/c8gc01220b Congfei Yao 1, 2, 3, 4 , Yucui Hou 4, 5, 6, 7 , Weize Wu 1, 2, 3, 4 , Shuhang Ren 1, 2, 3, 4 , Hui Liu 1, 2, 3, 4
Green Chemistry ( IF 9.8 ) Pub Date : 2018-06-05 , DOI: 10.1039/c8gc01220b Congfei Yao 1, 2, 3, 4 , Yucui Hou 4, 5, 6, 7 , Weize Wu 1, 2, 3, 4 , Shuhang Ren 1, 2, 3, 4 , Hui Liu 1, 2, 3, 4
Affiliation
|
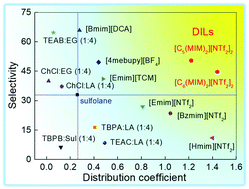
The separation of aromatics from aliphatics is challenging and energy-consuming because of the very close boiling points among these compounds and the possibility of forming azeotropes. In this work, we designed two imidazolium-based dicationic ionic liquids, [C5(MIM)2][NTf2]2 (DIL1) and [C6(MIM)2][NTf2]2 (DIL2), and found that they could highly efficiently extract aromatics from aliphatics. Three different aromatic and aliphatic mixtures, toluene/n-heptane, benzene/n-hexane and benzene/cyclohexane, were used to investigate the universality of DILs. The extractive performance of both DILs was compared with those of other promising ILs and sulfolane. The mixtures of toluene and aliphatics with different chain lengths were also investigated. The results showed that the two DILs had relatively high selectivity and distribution coefficients for different aromatic and aliphatic mixtures. At 30 °C, the highest values of selectivity and distribution coefficient for toluene/n-heptane, benzene/n-hexane and benzene/cyclohexane systems were 50.4, 64.8, and 24.9 and 1.44, 2.44, and 2.25, respectively. The performance of DILs at 30 °C was better than that at 40 °C. Besides, a higher selectivity could be obtained when toluene is used with a longer chain aliphatic, such as a selectivity of 61.4 could be obtained when the aliphatic was n-nonane. The study of the physicochemical properties of DILs showed that they were more stable than the previous ones. In a word, the two DILs were evaluated as potential solvents for the separation of aromatics from aliphatics.
中文翻译:

基于咪唑鎓的离子型离子液体:高效萃取剂,用于从脂肪族化合物中分离芳香族化合物
从脂肪族化合物中分离芳香族化合物具有挑战性且耗能,因为这些化合物之间的沸点非常接近,并且有可能形成共沸物。在这项工作中,我们设计了两种基于咪唑鎓的离子型离子液体[C 5(MIM)2 ] [NTf 2 ] 2(DIL1)和[C 6(MIM)2 ] [NTf 2 ] 2(DIL2),然后发现他们可以高效地从脂肪族中提取芳香族化合物。三种不同的芳香族和脂肪族混合物,甲苯/正庚烷,苯/ n-己烷和苯/环己烷用于研究DIL的通用性。将两种DIL的萃取性能与其他有前途的IL和环丁砜的萃取性能进行了比较。还研究了具有不同链长的甲苯和脂肪族化合物的混合物。结果表明,两种DIL对不同的芳香族和脂肪族混合物具有较高的选择性和分布系数。在30℃下,选择性和分配系数为甲苯的最高值和/ Ñ -庚烷,苯/ Ñ-己烷和苯/环己烷体系分别为50.4、64.8和24.9,以及1.44、2.44和2.25。DIL在30°C时的性能要好于40°C。此外,当甲苯与较长链的脂肪族化合物一起使用时,可以获得更高的选择性,例如当脂肪族化合物为正壬烷时,可以达到61.4的选择性。对DILs的理化性质的研究表明,它们比以前的DILs更稳定。简而言之,两个DIL被评估为潜在的溶剂,用于从脂肪族化合物中分离出芳香族化合物。
更新日期:2018-07-02
中文翻译:

基于咪唑鎓的离子型离子液体:高效萃取剂,用于从脂肪族化合物中分离芳香族化合物
从脂肪族化合物中分离芳香族化合物具有挑战性且耗能,因为这些化合物之间的沸点非常接近,并且有可能形成共沸物。在这项工作中,我们设计了两种基于咪唑鎓的离子型离子液体[C 5(MIM)2 ] [NTf 2 ] 2(DIL1)和[C 6(MIM)2 ] [NTf 2 ] 2(DIL2),然后发现他们可以高效地从脂肪族中提取芳香族化合物。三种不同的芳香族和脂肪族混合物,甲苯/正庚烷,苯/ n-己烷和苯/环己烷用于研究DIL的通用性。将两种DIL的萃取性能与其他有前途的IL和环丁砜的萃取性能进行了比较。还研究了具有不同链长的甲苯和脂肪族化合物的混合物。结果表明,两种DIL对不同的芳香族和脂肪族混合物具有较高的选择性和分布系数。在30℃下,选择性和分配系数为甲苯的最高值和/ Ñ -庚烷,苯/ Ñ-己烷和苯/环己烷体系分别为50.4、64.8和24.9,以及1.44、2.44和2.25。DIL在30°C时的性能要好于40°C。此外,当甲苯与较长链的脂肪族化合物一起使用时,可以获得更高的选择性,例如当脂肪族化合物为正壬烷时,可以达到61.4的选择性。对DILs的理化性质的研究表明,它们比以前的DILs更稳定。简而言之,两个DIL被评估为潜在的溶剂,用于从脂肪族化合物中分离出芳香族化合物。



























 京公网安备 11010802027423号
京公网安备 11010802027423号