当前位置:
X-MOL 学术
›
Chem. Asian J.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Kinetics of Spontaneous Bimetallization between Silver and Noble Metal Nanoparticles
Chemistry - An Asian Journal ( IF 3.5 ) Pub Date : 2018-06-25 , DOI: 10.1002/asia.201800633 Kazutaka Hirakawa 1 , Tetsuya Kaneko 1 , Naoki Toshima 2
Chemistry - An Asian Journal ( IF 3.5 ) Pub Date : 2018-06-25 , DOI: 10.1002/asia.201800633 Kazutaka Hirakawa 1 , Tetsuya Kaneko 1 , Naoki Toshima 2
Affiliation
|
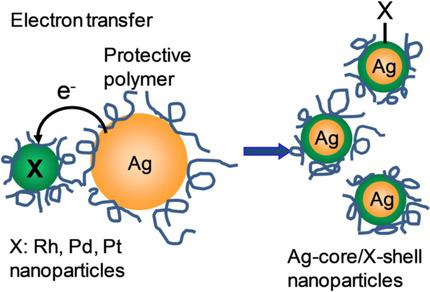
A physical mixture of polymer‐protected Ag nanoparticles and Rh, Pd, or Pt nanoparticles spontaneously forms Ag‐core bimetallic nanoparticles. The formed nanoparticles were smaller than the parent Ag nanoparticles. In the initial process of this reaction, the surface plasmon absorption of Ag nanoparticles diminished and then almost ceased within one hour. Within several minutes, the decrease in Ag surface plasmon absorption could be analyzed by second‐order reaction. This reaction was accelerated with an increase of temperature and the energy gap in the Fermi level between Ag and the other metals. The activation energy (Ea) of this reaction could be determined. An electron transfer reaction from Ag to other metal nanoparticles was proposed as the initial interaction between these metal nanoparticles because the Fermi level of Ag is relatively high, and the electron transfer is possible in terms of energy. The Marcus plot between the rate constant and the driving force, roughly estimated from the work function of metals, and the observed Ea values reasonably explained the proposed electron transfer mechanism.
中文翻译:

银与贵金属纳米颗粒之间自发双金属化的动力学
聚合物保护的银纳米颗粒和 Rh、Pd 或 Pt 纳米颗粒的物理混合物自发形成银核双金属纳米颗粒。形成的纳米颗粒比母体银纳米颗粒更小。在该反应的初始过程中,Ag纳米粒子的表面等离子体吸收减弱,然后在一小时内几乎停止。几分钟内,可以通过二级反应分析银表面等离子体吸收的减少。随着温度的升高和银与其他金属之间费米能级能隙的增加,该反应加速。可以测定该反应的活化能( E a )。提出从Ag到其他金属纳米颗粒的电子转移反应作为这些金属纳米颗粒之间的初始相互作用,因为Ag的费米能级相对较高,并且电子转移在能量方面是可能的。速率常数和驱动力之间的马库斯图(根据金属的功函数粗略估计)和观察到的E a值合理地解释了所提出的电子转移机制。
更新日期:2018-06-25
中文翻译:

银与贵金属纳米颗粒之间自发双金属化的动力学
聚合物保护的银纳米颗粒和 Rh、Pd 或 Pt 纳米颗粒的物理混合物自发形成银核双金属纳米颗粒。形成的纳米颗粒比母体银纳米颗粒更小。在该反应的初始过程中,Ag纳米粒子的表面等离子体吸收减弱,然后在一小时内几乎停止。几分钟内,可以通过二级反应分析银表面等离子体吸收的减少。随着温度的升高和银与其他金属之间费米能级能隙的增加,该反应加速。可以测定该反应的活化能( E a )。提出从Ag到其他金属纳米颗粒的电子转移反应作为这些金属纳米颗粒之间的初始相互作用,因为Ag的费米能级相对较高,并且电子转移在能量方面是可能的。速率常数和驱动力之间的马库斯图(根据金属的功函数粗略估计)和观察到的E a值合理地解释了所提出的电子转移机制。











































 京公网安备 11010802027423号
京公网安备 11010802027423号