当前位置:
X-MOL 学术
›
ChemMedChem
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Efficient Synthesis of Immunomodulatory Drug Analogues Enables Exploration of Structure-Degradation Relationships.
ChemMedChem ( IF 3.6 ) Pub Date : 2018-07-04 , DOI: 10.1002/cmdc.201800271 George M Burslem 1 , Philipp Ottis 1 , Saul Jaime-Figueroa 1 , Alicia Morgan 2 , Philipp M Cromm 1 , Momar Toure 1 , Craig M Crews 1, 3
ChemMedChem ( IF 3.6 ) Pub Date : 2018-07-04 , DOI: 10.1002/cmdc.201800271 George M Burslem 1 , Philipp Ottis 1 , Saul Jaime-Figueroa 1 , Alicia Morgan 2 , Philipp M Cromm 1 , Momar Toure 1 , Craig M Crews 1, 3
Affiliation
|
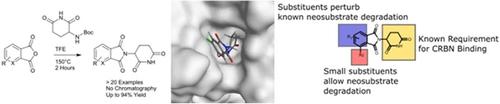
The immunomodulatory drugs (IMiDs) thalidomide, pomalidomide, and lenalidomide have been approved for the treatment of multiple myeloma for many years. Recently, their use as E3 ligase recruiting elements for small-molecule-induced protein degradation has led to a resurgence in interest in IMiD synthesis and functionalization. Traditional IMiD synthesis follows a stepwise route with multiple purification steps. Herein we describe a novel one-pot synthesis without purification that provides rapid access to a multitude of IMiD analogues. Binding studies with the IMiD target protein cereblon (CRBN) reveals a narrow structure-activity relationship with only a few compounds showing sub-micromolar binding affinity in the range of pomalidomide and lenalidomide. However, anti-proliferative activity as well as Aiolos degradation could be identified for two IMiD analogues. This study provides useful insight into the structure-degradation relationships for molecules of this type as well as a rapid and robust method for IMiD synthesis.
中文翻译:

免疫调节药物类似物的有效合成使得结构-降解关系的探索成为可能。
免疫调节药物 (IMiD) 沙利度胺、泊马度胺和来那度胺已被批准用于治疗多发性骨髓瘤多年。最近,它们作为 E3 连接酶招募元件用于小分子诱导的蛋白质降解,引起了人们对 IMiD 合成和功能化的兴趣重新兴起。传统的 IMiD 合成遵循具有多个纯化步骤的逐步路线。在此,我们描述了一种无需纯化的新型一锅合成法,可快速获得多种 IMiD 类似物。 IMiD 靶蛋白 cereblon (CRBN) 的结合研究揭示了狭窄的结构活性关系,仅少数化合物在泊马度胺和来那度胺范围内显示出亚微摩尔结合亲和力。然而,可以鉴定两种 IMiD 类似物的抗增殖活性以及 Aiolos 降解作用。这项研究为了解此类分子的结构-降解关系提供了有用的见解,并为 IMiD 合成提供了一种快速而可靠的方法。
更新日期:2018-07-04
中文翻译:

免疫调节药物类似物的有效合成使得结构-降解关系的探索成为可能。
免疫调节药物 (IMiD) 沙利度胺、泊马度胺和来那度胺已被批准用于治疗多发性骨髓瘤多年。最近,它们作为 E3 连接酶招募元件用于小分子诱导的蛋白质降解,引起了人们对 IMiD 合成和功能化的兴趣重新兴起。传统的 IMiD 合成遵循具有多个纯化步骤的逐步路线。在此,我们描述了一种无需纯化的新型一锅合成法,可快速获得多种 IMiD 类似物。 IMiD 靶蛋白 cereblon (CRBN) 的结合研究揭示了狭窄的结构活性关系,仅少数化合物在泊马度胺和来那度胺范围内显示出亚微摩尔结合亲和力。然而,可以鉴定两种 IMiD 类似物的抗增殖活性以及 Aiolos 降解作用。这项研究为了解此类分子的结构-降解关系提供了有用的见解,并为 IMiD 合成提供了一种快速而可靠的方法。











































 京公网安备 11010802027423号
京公网安备 11010802027423号