当前位置:
X-MOL 学术
›
ChemistrySelect
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Iodotriflamdation vs. Electrophilic Aromatic Iodination in the Reaction of N‐Phenyltriflamide with Alkenes
ChemistrySelect ( IF 1.9 ) Pub Date : 2018-06-04 , DOI: 10.1002/slct.201801379 Vera V. Astakhova 1 , Mikhail Yu. Moskalik 1 , Anton S. Ganin 1 , Irina V. Sterkhova 1 , Bagrat A. Shainyan 1
ChemistrySelect ( IF 1.9 ) Pub Date : 2018-06-04 , DOI: 10.1002/slct.201801379 Vera V. Astakhova 1 , Mikhail Yu. Moskalik 1 , Anton S. Ganin 1 , Irina V. Sterkhova 1 , Bagrat A. Shainyan 1
Affiliation
|
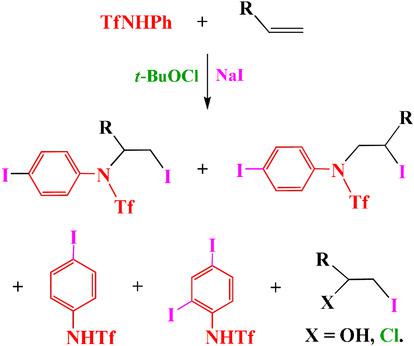
Reaction of N‐phenyltriflamide with styrene, p‐chlorostyrene, vinylcyclohexane and α‐methylstyrene in oxidative system (t‐BuOCl + NaI) gives three types of products: (i) of aromatic iodination of the reagent – N‐(4‐iodophenyl)triflamide and N‐(2,4‐diiodophenyl)triflamide; (ii) of oxidative addition of the iodinated reagent to the substrate with different regioselectivity – N‐[2‐iodo‐1‐(aryl)ethyl]‐N‐(4‐iodophenyl)triflamides or N‐(2‐cyclohexyl‐2‐iodoethyl)‐N‐(4‐iodophenyl)triflamide; (iii) of chloro(oxy)iodination of the substrate – 2‐iodo‐1‐phenylethanol, 1‐chloro‐4‐(1‐chloro‐2‐iodoethyl)benzene, 1‐cyclohexyl‐2‐iodoethanol and 1‐iodo‐2‐phenylpropan‐2‐ol. Different regioselectivity of iodosulfonamidation for different alkenes and the competition between electrophilic aromatic iodination and iodotriflamidation are explained and the mechanism is proposed based on the results of quantum chemical calculations and the NBO analysis.
中文翻译:

N-苯基三氟化物与烯烃反应中的碘三火焰化与亲电子芳香碘化
N-苯基三氟化物与氧化体系中的苯乙烯,对氯苯乙烯,乙烯基环己烷和α-甲基苯乙烯的反应(t-BuOCl + NaI)产生三种类型的产物:(i)试剂的芳族碘化– N-(4-碘苯基)三氟化物和N-(2,4-二碘苯基)三氟化物; (ii)将碘化试剂氧化添加到具有不同区域选择性的底物上-N- [2-碘-1-(芳基)乙基] -N-(4-碘苯基)三氟化物或N-(2-环己基-2-碘乙基)-N-(4-碘苯基)三氟化物; (iii)底物的氯(氧)碘化反应-2-碘-1--1-苯乙醇,1-氯-4-(1-氯-2-碘乙基)苯,1-环己基-2-碘乙醇和1-碘2-苯基丙烷-2-醇
更新日期:2018-06-04
中文翻译:

N-苯基三氟化物与烯烃反应中的碘三火焰化与亲电子芳香碘化
N-苯基三氟化物与氧化体系中的苯乙烯,对氯苯乙烯,乙烯基环己烷和α-甲基苯乙烯的反应(t-BuOCl + NaI)产生三种类型的产物:(i)试剂的芳族碘化– N-(4-碘苯基)三氟化物和N-(2,4-二碘苯基)三氟化物; (ii)将碘化试剂氧化添加到具有不同区域选择性的底物上-N- [2-碘-1-(芳基)乙基] -N-(4-碘苯基)三氟化物或N-(2-环己基-2-碘乙基)-N-(4-碘苯基)三氟化物; (iii)底物的氯(氧)碘化反应-2-碘-1--1-苯乙醇,1-氯-4-(1-氯-2-碘乙基)苯,1-环己基-2-碘乙醇和1-碘2-苯基丙烷-2-醇











































 京公网安备 11010802027423号
京公网安备 11010802027423号