当前位置:
X-MOL 学术
›
ChemistrySelect
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Aqueous Suzuki‐Miyaura Reaction with 0.6 Equiv. of Base: Green and Efficient Access to Biaryls and Unsymmetrical Terphenyls
ChemistrySelect ( IF 2.1 ) Pub Date : 2018-06-04 , DOI: 10.1002/slct.201800946 Xinmin Li 1 , Yong Teng 1 , Fangfang Feng 1 , Qinghong Hu 1 , Zeli Yuan 1
ChemistrySelect ( IF 2.1 ) Pub Date : 2018-06-04 , DOI: 10.1002/slct.201800946 Xinmin Li 1 , Yong Teng 1 , Fangfang Feng 1 , Qinghong Hu 1 , Zeli Yuan 1
Affiliation
|
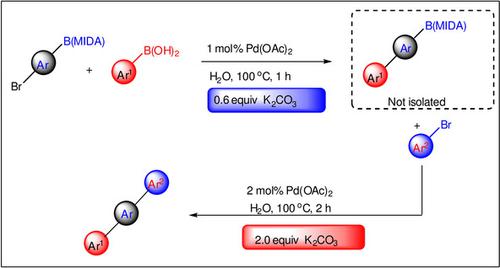
A ligand‐free palladium‐catalyzed Suzuki–Miyaura reaction in water with 0.6 equiv. of base is described. This method enables the preparation of a variety of biaryls in high yields. Additionally, using bromo‐N‐methyliminodiacetic acid (MIDA) boronates as building block, one‐pot double Suzuki–Miyaura reaction has been achieved by controlling the amount of base, and various unsymmetrical terphenyls were prepared in moderate to good yields.
中文翻译:

0.6当量的Suzuki-Miyaura水溶液反应 基数:绿色高效地获得联芳基和不对称三联苯
0.6当量的水中无配体的钯催化的Suzuki-Miyaura反应。描述了基数。该方法能够以高收率制备各种联芳基。此外,使用溴化N-甲基亚氨基二乙酸(MIDA)硼酸盐作为结构单元,可以通过控制碱的用量实现一锅双铃木-宫浦双反应,并以中等至良好的收率制备了各种不对称的三联苯。
更新日期:2018-06-04
中文翻译:

0.6当量的Suzuki-Miyaura水溶液反应 基数:绿色高效地获得联芳基和不对称三联苯
0.6当量的水中无配体的钯催化的Suzuki-Miyaura反应。描述了基数。该方法能够以高收率制备各种联芳基。此外,使用溴化N-甲基亚氨基二乙酸(MIDA)硼酸盐作为结构单元,可以通过控制碱的用量实现一锅双铃木-宫浦双反应,并以中等至良好的收率制备了各种不对称的三联苯。



























 京公网安备 11010802027423号
京公网安备 11010802027423号