Bioorganic Chemistry ( IF 5.1 ) Pub Date : 2018-06-05 , DOI: 10.1016/j.bioorg.2018.06.003 Mahmoud S. Abdelbaset , Gamal El-Din A. Abuo-Rahma , Mostafa H. Abdelrahman , Mohamed Ramadan , Bahaa G.M. Youssif , Syed Nasir Abbas Bukhari , Mamdouh F.A. Mohamed , Mohamed Abdel-Aziz
|
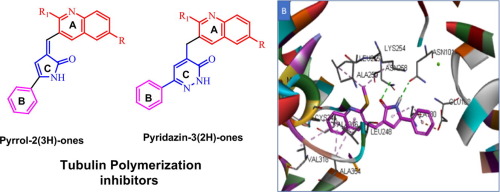
A novel quinolinyl pyrrolone and quinolinyl pyridazinone derivatives has been synthesized and characterized using different spectroscopic and elemental analysis techniques. Most of the target compounds displayed promising antiproliferative activity; In general, the pyrrolone derivatives 4a-f exhibited higher antiproliferative activity than their corresponding pyridazinone. The pyrrolone 4f showed outstanding antiproliferative activity with moderate selectivity against CNS and renal cancer with selectivity ratio of 3.49 and 3.56, respectively. Compound 4e and 5d experienced tubulin polymerization inhibitory activity comparable to that of vincristine while 4c, 4e and 4d showed good BRAF kinase inhibition compared to Erlotinib. Docking of compound 4e into colchicine binding site and biological assay results revealed that these compounds act mainly through tubulin polymerization inhibitory mechanism and can exhibit pre G1 apoptosis and cell cycle arrest at G2/M phase.
中文翻译:

新型吡咯-2(3 H)-酮和哒嗪-3(2 H)-酮带有喹啉骨架作为抗增殖微管蛋白聚合抑制剂
已经合成了新颖的喹啉基吡咯烷酮和喹啉基哒嗪酮衍生物,并使用不同的光谱和元素分析技术对其进行了表征。大多数目标化合物显示出有希望的抗增殖活性。通常,吡咯烷酮衍生物4a-f显示出比其相应的哒嗪酮更高的抗增殖活性。吡咯烷酮4f具有出色的抗增殖活性,对中枢神经系统和肾癌的选择性中等,选择性比分别为3.49和3.56。化合物4e和5d的微管蛋白聚合抑制活性与长春新碱相当,而4c,4e和4d与厄洛替尼相比,具有良好的BRAF激酶抑制作用。化合物4e对接秋水仙碱结合位点和生物学分析结果表明,这些化合物主要通过微管蛋白聚合抑制机制起作用,并且可以显示G1前期细胞凋亡和细胞周期停滞在G2 / M期。



























 京公网安备 11010802027423号
京公网安备 11010802027423号