当前位置:
X-MOL 学术
›
J. Phys. Chem. Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Probing the Reaction Interface in Li–Oxygen Batteries Using Dynamic Electrochemical Impedance Spectroscopy: Discharge–Charge Asymmetry in Reaction Sites and Electronic Conductivity
The Journal of Physical Chemistry Letters ( IF 4.8 ) Pub Date : 2018-06-04 00:00:00 , DOI: 10.1021/acs.jpclett.8b01351 Jun Huang 1, 2, 3 , Bo Tong 2 , Zhe Li 3 , Tao Zhou 1 , Jianbo Zhang 3 , Zhangquan Peng 2
The Journal of Physical Chemistry Letters ( IF 4.8 ) Pub Date : 2018-06-04 00:00:00 , DOI: 10.1021/acs.jpclett.8b01351 Jun Huang 1, 2, 3 , Bo Tong 2 , Zhe Li 3 , Tao Zhou 1 , Jianbo Zhang 3 , Zhangquan Peng 2
Affiliation
|
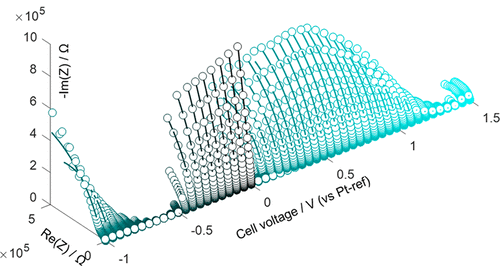
Fundamental questions concerning the reaction interface in Li–O2 batteries, including where reactions occur and discharge–charge asymmetries come from, have stimulated a flurry of investigations; nevertheless, heated debates still prevail. Dynamic electrochemical impedance spectroscopy (EIS) is employed here to probe the reaction interface in a Li–O2 battery under potentiostatic and galvanostatic modes. Two impedance semicircles are identified during discharge with the high- and the low-frequency ones related to the Li2O2 film and the oxygen reduction reaction (ORR), respectively. However, upon triggering the oxygen evolution reaction (OER), only one semicircle is observed, implying that the reaction interface changes. Combining qualitative analysis on the EIS structure and quantitative information obtained from model fitting reveals that the ORR occurs on the Li2O2–electrolyte interface during discharge and the OER occurs on the electrode surface during charge. In addition, it is found that the electronic conductivity of Li2O2 is higher at oxidative potentials (charge) than reductive potentials (discharge). Discharge–charge differences in the reaction interface and the electronic conductivity reported here expand the scope of discharge–charge asymmetries of Li–O2 batteries.
中文翻译:

使用动态电化学阻抗谱探测锂氧电池中的反应界面:反应部位的放电-电荷不对称性和电子电导率
有关Li-O 2电池反应界面的基本问题,包括反应发生的地方和放电-充电不对称的来源,引起了人们的大量研究。然而,激烈的辩论仍然占上风。在这里,采用动态电化学阻抗谱(EIS)来探测Li–O 2电池在恒电位和恒电流模式下的反应界面。在放电过程中识别出两个阻抗半圆,分别是与Li 2 O 2有关的高频和低频半圆。膜和氧还原反应(ORR)。但是,在触发放氧反应(OER)时,仅观察到一个半圆,这表明反应界面发生了变化。结合对EIS结构的定性分析和从模型拟合中获得的定量信息,可以发现,放电期间ORR发生在Li 2 O 2-电解质界面上,充电时OER发生在电极表面上。另外,发现Li 2 O 2的电子电导率在氧化电位(电荷)处比在还原电位(放电处)高。反应界面中的充放电差异和此处报道的电子电导率扩大了Li–O 2电池的充放电不对称性范围。
更新日期:2018-06-04
中文翻译:

使用动态电化学阻抗谱探测锂氧电池中的反应界面:反应部位的放电-电荷不对称性和电子电导率
有关Li-O 2电池反应界面的基本问题,包括反应发生的地方和放电-充电不对称的来源,引起了人们的大量研究。然而,激烈的辩论仍然占上风。在这里,采用动态电化学阻抗谱(EIS)来探测Li–O 2电池在恒电位和恒电流模式下的反应界面。在放电过程中识别出两个阻抗半圆,分别是与Li 2 O 2有关的高频和低频半圆。膜和氧还原反应(ORR)。但是,在触发放氧反应(OER)时,仅观察到一个半圆,这表明反应界面发生了变化。结合对EIS结构的定性分析和从模型拟合中获得的定量信息,可以发现,放电期间ORR发生在Li 2 O 2-电解质界面上,充电时OER发生在电极表面上。另外,发现Li 2 O 2的电子电导率在氧化电位(电荷)处比在还原电位(放电处)高。反应界面中的充放电差异和此处报道的电子电导率扩大了Li–O 2电池的充放电不对称性范围。











































 京公网安备 11010802027423号
京公网安备 11010802027423号