Bioorganic Chemistry ( IF 4.5 ) Pub Date : 2018-06-04 , DOI: 10.1016/j.bioorg.2018.06.005 Samia Chekir , Meriem Debbabi , Anne Regazzetti , Delphine Dargère , Olivier Laprévote , Hichem Ben Jannet , Rafik Gharbi
|
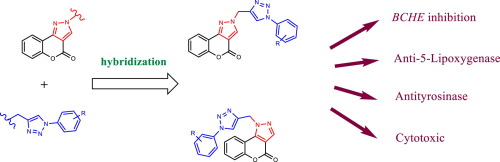
A series of new 1,2,3-triazole linked coumarinopyrazole conjugates 4a-e and 5a-e have been synthesized via the Copper(I)-catalysed Alkyne-Azide Cycloaddition (CuAAC). Going through the reaction of compound 2 with the 3-propargyl bromide gave a mixture of propargylated regioisomers 3 + 3′ used as a dipolarophile to access to triazoles 4a-e and 5a-e. The structures of the prepared cycloadducts were determined by 1H, 13C and 2D-NMR techniques and by HRMS analysis. All the synthesized derivatives have been evaluated for their anticholinesterase, anti-5-lipoxygenase, anti-tyrosinase, and cytotoxic activities.
中文翻译:

新型1,2,3-三唑连接香豆素吡唑共轭物作为有效的抗胆碱酯酶,抗5-脂氧合酶,抗酪氨酸酶和抗癌药的设计,合成和生物学评价
通过铜(I)催化的炔-叠氮化物环加成反应(CuAAC)合成了一系列新的1,2,3-三唑连接的香豆素吡唑共轭物4a-e和5a-e。通过化合物2与3-炔丙基溴的反应,得到炔丙基化的区域异构体3 + 3'的混合物,其用作双极性亲和剂以得到三唑4a-e和5a-e。制备的环加合物的结构通过1 H,13 C和2D-NMR技术以及HRMS分析确定。已经评估了所有合成衍生物的抗胆碱酯酶,抗5-脂氧合酶,抗酪氨酸酶和细胞毒性活性。











































 京公网安备 11010802027423号
京公网安备 11010802027423号