当前位置:
X-MOL 学术
›
J. Chem. Eng. Data
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Solubility of Sulfachloropyridazine in Pure and Binary Solvent Mixtures and Investigation of Intermolecular Interactions
Journal of Chemical & Engineering Data ( IF 2.0 ) Pub Date : 2018-06-04 , DOI: 10.1021/acs.jced.7b01134 Rongrong Li 1 , Sangfei Ye 1 , Yongfei Chen 1 , Ming Jiang 2 , Yanxian Jin 1 , Wenping Jia 1 , Xiaoying Chen 1 , Shiyun Zhan 1 , Deman Han 1
Journal of Chemical & Engineering Data ( IF 2.0 ) Pub Date : 2018-06-04 , DOI: 10.1021/acs.jced.7b01134 Rongrong Li 1 , Sangfei Ye 1 , Yongfei Chen 1 , Ming Jiang 2 , Yanxian Jin 1 , Wenping Jia 1 , Xiaoying Chen 1 , Shiyun Zhan 1 , Deman Han 1
Affiliation
|
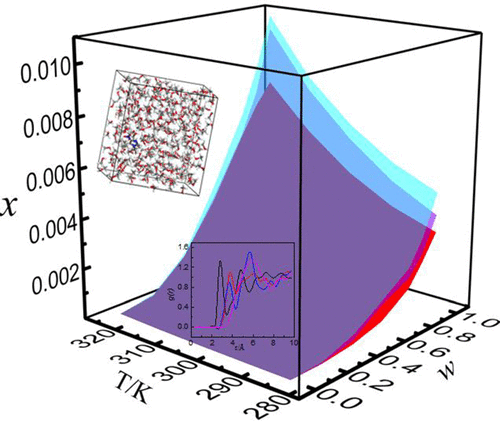
The solubility of sulfachloropyridazine in pure methanol, ethanol, isopropanol, 1-butanol, water, acetonitrile, ethyl acetate, acetone, 1,4-dioxane and (methanol + water), (ethanol + water), and (isopropanol + water) binary solvent mixtures was determined by using a isothermal saturation method with temperatures ranging from (283.15 to 323.15) K. The descending order of the solubility in pure solvents was as follows: 1,4-dioxane > acetone > acetonitrile > ethyl acetate > methanol > ethanol >1-butanol > isopropanol > water. In three mixed solvents, the solubility of sulfachloropyridazine increased with increasing temperature and mass fraction of alcohol. At the same mass fraction of methanol, ethanol, or isopropanol and temperature, the solubility of sulfachloropyridazine in (methanol + water) was greater than that in the other mixed solvents. The obtained solubility data were correlated with modified Apelblat equation and Jouyban–Acree model. The results of correlation showed good agreement with experimental data; the largest values of relative average deviations and the root-mean-square deviations between the experimental and calculated solubilities were 3.61 × 10–2 and 6.37 × 10–4, respectively. Furthermore, molecular dynamics simulation was carried out to explain the dissolving ability of the model compound.
中文翻译:

磺胺氯哒嗪在纯和二元溶剂混合物中的溶解度和分子间相互作用的研究
磺胺氯哒嗪在纯甲醇,乙醇,异丙醇,1-丁醇,水,乙腈,乙酸乙酯,丙酮,1,4-二恶烷和(甲醇+水),(乙醇+水)和(异丙醇+水)二元化合物中的溶解度通过使用等温饱和方法确定溶剂混合物,温度范围为(283.15至323.15)K。在纯溶剂中的溶解度降序如下:1,4-二恶烷>丙酮>乙腈>乙酸乙酯>甲醇>乙醇> 1-丁醇>异丙醇>水。在三种混合溶剂中,磺胺氯哒嗪的溶解度随温度和醇质量分数的增加而增加。在相同质量分数的甲醇,乙醇或异丙醇和温度下,磺胺氯哒嗪在(甲醇+水)中的溶解度大于在其他混合溶剂中的溶解度。获得的溶解度数据与修正的Apelblat方程和Jouyban–Acree模型相关。相关结果与实验数据吻合良好。实验和计算的溶解度之间的相对平均偏差和均方根偏差的最大值为3.61×10–2和6.37×10 –4。此外,进行了分子动力学模拟以解释模型化合物的溶解能力。
更新日期:2018-06-05
中文翻译:

磺胺氯哒嗪在纯和二元溶剂混合物中的溶解度和分子间相互作用的研究
磺胺氯哒嗪在纯甲醇,乙醇,异丙醇,1-丁醇,水,乙腈,乙酸乙酯,丙酮,1,4-二恶烷和(甲醇+水),(乙醇+水)和(异丙醇+水)二元化合物中的溶解度通过使用等温饱和方法确定溶剂混合物,温度范围为(283.15至323.15)K。在纯溶剂中的溶解度降序如下:1,4-二恶烷>丙酮>乙腈>乙酸乙酯>甲醇>乙醇> 1-丁醇>异丙醇>水。在三种混合溶剂中,磺胺氯哒嗪的溶解度随温度和醇质量分数的增加而增加。在相同质量分数的甲醇,乙醇或异丙醇和温度下,磺胺氯哒嗪在(甲醇+水)中的溶解度大于在其他混合溶剂中的溶解度。获得的溶解度数据与修正的Apelblat方程和Jouyban–Acree模型相关。相关结果与实验数据吻合良好。实验和计算的溶解度之间的相对平均偏差和均方根偏差的最大值为3.61×10–2和6.37×10 –4。此外,进行了分子动力学模拟以解释模型化合物的溶解能力。











































 京公网安备 11010802027423号
京公网安备 11010802027423号