当前位置:
X-MOL 学术
›
J. Chem. Eng. Data
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Solubility and Preferential Solvation of 3-Nitrobenzonitrile in Binary Solvent Mixtures of Ethyl Acetate Plus (Methanol, Ethanol, n-Propanol, and Isopropyl Alcohol)
Journal of Chemical & Engineering Data ( IF 2.6 ) Pub Date : 2018-06-04 , DOI: 10.1021/acs.jced.8b00230 Min Zheng , Jiao Chen , Renjie Xu , Gaoquan Chen , Yang Cong , Hongkun Zhao
Journal of Chemical & Engineering Data ( IF 2.6 ) Pub Date : 2018-06-04 , DOI: 10.1021/acs.jced.8b00230 Min Zheng , Jiao Chen , Renjie Xu , Gaoquan Chen , Yang Cong , Hongkun Zhao
|
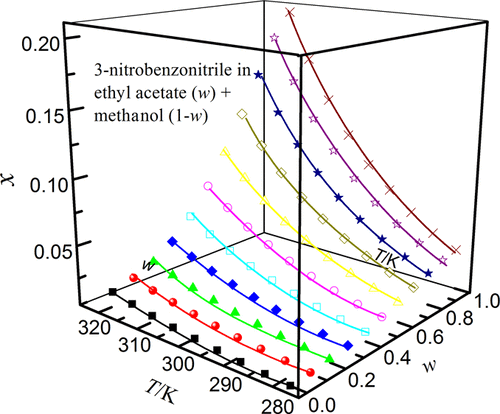
The solubilities of 3-nitrobenzonitrile in solvent mixtures of ethyl acetate (1) + methanol (ethanol, n-propanol or isopropyl alcohol) (2) determined over the temperature range from 278.15 to 318.15 K under atmospheric pressure (101.1 kPa) with the isothermal dissolution equilibrium method were reported. They increased with a rise of temperature and mass fraction of ethyl acetate, and the largest solubility value was observed in neat ethyl acetate for all the binary mixtures investigated. The temperature and solvent composition dependence of 3-nitrobenzonitrile solubility was analyzed through the Jouyban–Acree, van’t Hoff–Jouyban–Acree, and Apelblat–Jouyban–Acree models acquiring average relative deviations lower than 1.57% and root-mean-square deviation lower than 11.52 × 10–4 for correlative investigations. In addition, the preferential solvation parameters (δx1,3) of 3-nitrobenzonitrile by ethyl acetate were determined from experimental solubility values by using the inverse Kirkwood–Buff integrals. It was found that alcohol preferentially solvated 3-nitrobenzonitrile in alcohol-rich mixtures while ethyl acetate forms local solvation shells in compositions from intermediate composition up to neat ethyl acetate. The former case was possibly due to the ordered structure of alcohol molecules around the apolar group of 3-nitrobenzonitrile, which was formed via hydrophobic hydration in alcohol-rich solutions.
中文翻译:

溶解度和在乙酸乙酯加(甲醇,乙醇,二元溶剂混合物-3-硝基苄腈的优先溶剂化Ñ丙醇,和异丙醇)
在278.15至318.15 K的温度范围内,大气压(101.1 kPa)等温下测定的3-硝基苄腈在乙酸乙酯(1)+甲醇(乙醇,正丙醇或异丙醇)(2)的溶剂混合物中的溶解度报道了溶解平衡法。它们随温度和乙酸乙酯质量分数的增加而增加,对于所有研究的二元混合物,在纯净乙酸乙酯中观察到最大的溶解度值。通过Jouyban–Acree,van't Hoff–Jouyban–Acree和Apelblat–Jouyban–Acree模型分析了3-硝基苯甲腈溶解度对温度和溶剂成分的依赖性,得出平均相对偏差低于1.57%和均方根偏差低于11.52×10 –4用于相关调查。此外,优先溶剂化参数(δ X 1,3)-3-硝基苄腈的由乙酸乙酯通过使用逆柯克伍德-巴夫积分从实验溶解度值确定。发现在富含醇的混合物中醇优先使3-硝基苄腈溶剂化,而乙酸乙酯在从中间组合物直至纯乙酸乙酯的组合物中形成局部溶剂化壳。前一种情况可能是由于在3-硝基苄腈的非极性基团周围的醇分子的有序结构,该结构是通过在富醇溶液中进行疏水水合而形成的。
更新日期:2018-06-05
中文翻译:

溶解度和在乙酸乙酯加(甲醇,乙醇,二元溶剂混合物-3-硝基苄腈的优先溶剂化Ñ丙醇,和异丙醇)
在278.15至318.15 K的温度范围内,大气压(101.1 kPa)等温下测定的3-硝基苄腈在乙酸乙酯(1)+甲醇(乙醇,正丙醇或异丙醇)(2)的溶剂混合物中的溶解度报道了溶解平衡法。它们随温度和乙酸乙酯质量分数的增加而增加,对于所有研究的二元混合物,在纯净乙酸乙酯中观察到最大的溶解度值。通过Jouyban–Acree,van't Hoff–Jouyban–Acree和Apelblat–Jouyban–Acree模型分析了3-硝基苯甲腈溶解度对温度和溶剂成分的依赖性,得出平均相对偏差低于1.57%和均方根偏差低于11.52×10 –4用于相关调查。此外,优先溶剂化参数(δ X 1,3)-3-硝基苄腈的由乙酸乙酯通过使用逆柯克伍德-巴夫积分从实验溶解度值确定。发现在富含醇的混合物中醇优先使3-硝基苄腈溶剂化,而乙酸乙酯在从中间组合物直至纯乙酸乙酯的组合物中形成局部溶剂化壳。前一种情况可能是由于在3-硝基苄腈的非极性基团周围的醇分子的有序结构,该结构是通过在富醇溶液中进行疏水水合而形成的。



























 京公网安备 11010802027423号
京公网安备 11010802027423号