当前位置:
X-MOL 学术
›
ACS Earth Space Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Can Formamide Be Formed on Interstellar Ice? An Atomistic Perspective
ACS Earth and Space Chemistry ( IF 2.9 ) Pub Date : 2018-06-04 00:00:00 , DOI: 10.1021/acsearthspacechem.7b00156 Albert Rimola 1 , Dimitrios Skouteris 2 , Nadia Balucani 3, 4, 5 , Cecilia Ceccarelli 5 , Joan Enrique-Romero 5 , Vianney Taquet 3 , Piero Ugliengo 6
ACS Earth and Space Chemistry ( IF 2.9 ) Pub Date : 2018-06-04 00:00:00 , DOI: 10.1021/acsearthspacechem.7b00156 Albert Rimola 1 , Dimitrios Skouteris 2 , Nadia Balucani 3, 4, 5 , Cecilia Ceccarelli 5 , Joan Enrique-Romero 5 , Vianney Taquet 3 , Piero Ugliengo 6
Affiliation
|
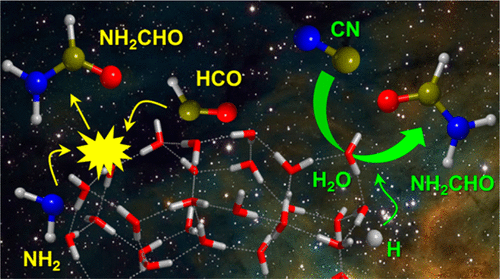
Interstellar formamide (NH2CHO) has recently attracted significant attention due to its potential role as a molecular building block in the formation of precursor biomolecules relevant for the origin of life. Its formation, whether on the surfaces of the interstellar grains or in the gas phase, is currently debated. The present article presents new theoretical computations based on quantum chemical and kinetic calculations on possible NH2CHO formation routes in water-rich amorphous ices, simulated by a 33 H2O molecule cluster. We have considered three possible routes. The first one refers to a scenario used in several current astrochemical models, that is, the radical–radical association reaction between NH2 and HCO. Our calculations show that formamide can indeed be formed, but in competition with formation of NH3 and CO through a direct H transfer process. The final outcome of the NH2 + HCO reactivity depends on the relative orientation of the two radicals on the ice surface. We also analyzed two other possibilities, suggested here for the first time: reaction of either HCN or CN with water molecules of the ice mantle. The reaction with HCN has been found to be characterized by large energy barriers and, therefore, cannot occur under the interstellar ice conditions. On the contrary, the reaction with the CN radical can occur, possibly leading through multiple steps to the formation of NH2CHO. For this reaction, water molecules of the ice act as catalytic active sites since they help the H transfers involved in the process, thus reducing the energy barriers (compared to the gas-phase analogous reaction). Additionally, we apply a statistical model to estimate the reaction rate coefficient when considering the cluster of 33 H2O molecules as an isolated moiety with respect to the surrounding environment (i.e., the rest of the ice). Our conclusion is that CN quickly reacts with a molecule of amorphous ice and that it can synthesize formamide, even though the efficiency of the NH2CHO formation is difficult to estimate as it depends on the unknown number of ice water active sites and the fine details of energy transfer through the ice body itself. Our results have two important consequences on the modeling of interstellar surface-chemistry. First, the H2O molecules of the ice, usually considered as an inert support in astrochemical models, can instead react with active radicals, like CN, forming more complex species, and can also act as catalysts by helping H transfer processes. Second, most of the involved intermediate steps toward formamide formation on the 33-H2O molecule cluster are so fast that it is unlikely that the energy released in each of them can be dispersed in the entire ice body of the grain. In other words, the system cannot be fully equilibrated at the grain temperature in each intermediate step, as assumed in all current models, because the localized energy can promote endothermic or high barrier processes in small portions of the ice before complete equilibration. The time scale of energy redistribution within the ice molecules, a poorly characterized process, should be explicitly accounted for if a realistic model of grain surface chemistry is pursued.
中文翻译:

可以在星际冰上形成甲酰胺吗?原子视角
星际甲酰胺(NH 2 CHO)由于其作为与生命起源相关的前体生物分子形成中的分子构件的潜在作用,最近引起了广泛的关注。无论是在星际颗粒的表面还是在气相中,其形成都受到争议。本文基于33 H 2 O分子簇模拟,提出了基于量子化学和动力学计算的新理论计算方法,该计算方法是在富水非晶冰中可能的NH 2 CHO形成路线的。我们考虑了三种可能的路线。第一个是指在几种当前的天体化学模型中使用的场景,即NH 2之间的自由基-自由基缔合反应和HCO。我们的计算表明确实可以形成甲酰胺,但是与通过直接的H转移过程形成NH 3和CO相竞争。NH 2 + HCO反应性的最终结果取决于两个自由基在冰面上的相对取向。我们还分析了另外两种可能性,这是首次在这里提出:HCN或CN与冰幔中水分子的反应。已发现与HCN的反应具有较大的能垒,因此在星际冰雪条件下不会发生。相反,可能发生与CN基团的反应,可能会通过多个步骤导致NH 2的形成CHO。对于该反应,冰中的水分子充当催化活性位点,因为它们帮助参与过程的H转移,从而减少了能垒(与气相类似反应相比)。此外,当考虑将33 H 2 O分子簇作为相对于周围环境(即冰的其余部分)的分离部分时,我们应用统计模型来估计反应速率系数。我们的结论是,即使NH 2的效率很高,CN也会迅速与无定形冰分子发生反应,并且可以合成甲酰胺。CHO的形成很难估计,因为它取决于未知数量的冰水活动位点以及通过冰体本身进行的能量传递的精细细节。我们的结果对星际表面化学的建模有两个重要的结果。首先,冰的H 2 O分子通常在天化学模型中被认为是一种惰性载体,它可以与活性自由基(如CN)反应,形成更复杂的物种,并且还可以通过帮助H转移过程充当催化剂。其次,大多数涉及在33-H 2上形成甲酰胺的中间步骤O分子簇是如此之快,以至于每个分子释放的能量不可能分散在谷物的整个冰体中。换句话说,如所有当前模型所假设的那样,无法在每个中间步骤中的谷物温度下完全平衡系统,因为局部能量可以在完全平衡之前促进小部分冰块中的吸热或高阻隔过程。如果追求真实的谷物表面化学模型,则应明确考虑冰分子内能量重新分布的时间尺度(一个表征较差的过程)。
更新日期:2018-06-04
中文翻译:

可以在星际冰上形成甲酰胺吗?原子视角
星际甲酰胺(NH 2 CHO)由于其作为与生命起源相关的前体生物分子形成中的分子构件的潜在作用,最近引起了广泛的关注。无论是在星际颗粒的表面还是在气相中,其形成都受到争议。本文基于33 H 2 O分子簇模拟,提出了基于量子化学和动力学计算的新理论计算方法,该计算方法是在富水非晶冰中可能的NH 2 CHO形成路线的。我们考虑了三种可能的路线。第一个是指在几种当前的天体化学模型中使用的场景,即NH 2之间的自由基-自由基缔合反应和HCO。我们的计算表明确实可以形成甲酰胺,但是与通过直接的H转移过程形成NH 3和CO相竞争。NH 2 + HCO反应性的最终结果取决于两个自由基在冰面上的相对取向。我们还分析了另外两种可能性,这是首次在这里提出:HCN或CN与冰幔中水分子的反应。已发现与HCN的反应具有较大的能垒,因此在星际冰雪条件下不会发生。相反,可能发生与CN基团的反应,可能会通过多个步骤导致NH 2的形成CHO。对于该反应,冰中的水分子充当催化活性位点,因为它们帮助参与过程的H转移,从而减少了能垒(与气相类似反应相比)。此外,当考虑将33 H 2 O分子簇作为相对于周围环境(即冰的其余部分)的分离部分时,我们应用统计模型来估计反应速率系数。我们的结论是,即使NH 2的效率很高,CN也会迅速与无定形冰分子发生反应,并且可以合成甲酰胺。CHO的形成很难估计,因为它取决于未知数量的冰水活动位点以及通过冰体本身进行的能量传递的精细细节。我们的结果对星际表面化学的建模有两个重要的结果。首先,冰的H 2 O分子通常在天化学模型中被认为是一种惰性载体,它可以与活性自由基(如CN)反应,形成更复杂的物种,并且还可以通过帮助H转移过程充当催化剂。其次,大多数涉及在33-H 2上形成甲酰胺的中间步骤O分子簇是如此之快,以至于每个分子释放的能量不可能分散在谷物的整个冰体中。换句话说,如所有当前模型所假设的那样,无法在每个中间步骤中的谷物温度下完全平衡系统,因为局部能量可以在完全平衡之前促进小部分冰块中的吸热或高阻隔过程。如果追求真实的谷物表面化学模型,则应明确考虑冰分子内能量重新分布的时间尺度(一个表征较差的过程)。











































 京公网安备 11010802027423号
京公网安备 11010802027423号