当前位置:
X-MOL 学术
›
ACS Photonics
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Metamaterials-Enhanced Infrared Spectroscopic Study of Nanoconfined Molecules by Plasmonics–Nanofluidics Hydrid Device
ACS Photonics ( IF 6.5 ) Pub Date : 2018-06-04 00:00:00 , DOI: 10.1021/acsphotonics.8b00398 Thu H. H. Le 1, 2 , Akihiro Morita 3, 4 , Kazuma Mawatari 2 , Takehiko Kitamori 2 , Takuo Tanaka 1, 5, 6
ACS Photonics ( IF 6.5 ) Pub Date : 2018-06-04 00:00:00 , DOI: 10.1021/acsphotonics.8b00398 Thu H. H. Le 1, 2 , Akihiro Morita 3, 4 , Kazuma Mawatari 2 , Takehiko Kitamori 2 , Takuo Tanaka 1, 5, 6
Affiliation
|
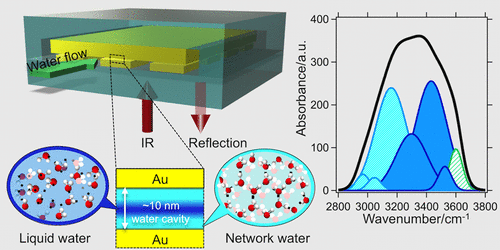
The behavior of molecules under nanoconfinement is crucial for understanding the chemical processes in biological and nanomaterial systems. We demonstrated here an infrared spectroscopic method to characterize the molecular structures of molecules confined in several tens of nanometer cavities by employing the plasmonics–nanofluidics hybrid device. This device consists of an array of metal nanostructures and a metal mirror separated by a nanofluidic cavity. Its configuration enables the confinement of both molecules and light energy as localized surface plasmons inside the physicochemically well-defined nanocavity. Exploiting the plasmons–molecular coupling, the vibrational modes of the nanoconfined molecules are selectively detected with a prominent sensitivity. Applying water as a proof-of-concept sample, we have successfully measured the infrared absorption characteristic and elucidated the molecular structures of water confined in a 10 nm cavity. They unveiled the presence of a strong H-bond network with respect to bulk water. Our method was also able to distinguish the subtle differences in the molecular structures, revealing the scaling behavior of confined water in the several tens of nanometer size regime. This effect is also found not being driven by the interaction with the interfaces; yet the constrained geometry itself promotes the intermolecular interactions of water and results in the modification of the H-bond network. This study has offered an ultrasensitive platform for in situ probing of the nanoconfined molecules and chemical reactions in their intact condition, and thus gives us a fundamental insight into the nanoconfinement effects.
中文翻译:

等离子-纳米流体氢化装置对纳米材料分子的超材料增强红外光谱研究
纳米约束下的分子行为对于理解生物和纳米材料系统中的化学过程至关重要。我们在这里展示了一种红外光谱方法,通过使用等离子-纳米流体混合装置来表征被限制在几十个纳米腔中的分子的分子结构。该装置由金属纳米结构阵列和被纳米流体腔隔开的金属镜组成。它的配置可以将分子和光能限制为在物理化学上定义明确的纳米腔体内的局部表面等离激元。利用等离激元-分子耦合,可以以显着的灵敏度选择性地检测纳米约束分子的振动模式。用水作为概念验证样品,我们已经成功地测量了红外吸收特性,并阐明了封闭在10 nm腔中的水的分子结构。他们揭露了与散装水有关的强大的氢键网络。我们的方法还能够区分分子结构中的细微差别,从而揭示了承压水在数十纳米尺寸范围内的结垢行为。还发现这种影响不是由与接口的交互作用所驱动的。然而,受约束的几何形状本身会促进水的分子间相互作用,并导致氢键网络的改性。这项研究为 他们揭露了与散装水有关的强大的氢键网络。我们的方法还能够区分分子结构中的细微差别,从而揭示了承压水在数十纳米尺寸范围内的结垢行为。还发现这种影响不是由与接口的交互作用所驱动的。然而,受约束的几何形状本身会促进水的分子间相互作用,并导致氢键网络的改性。这项研究为 他们揭露了与散装水有关的强大的氢键网络。我们的方法还能够区分分子结构中的细微差别,从而揭示了承压水在数十纳米尺寸范围内的结垢行为。还发现这种影响不是由与接口的交互作用所驱动的。然而,受约束的几何形状本身会促进水的分子间相互作用,并导致氢键网络的改性。这项研究为 然而,受约束的几何形状本身会促进水的分子间相互作用,并导致氢键网络的改性。这项研究为 然而,受约束的几何形状本身会促进水的分子间相互作用,并导致氢键网络的改性。这项研究为原位探测纳米约束分子及其完整状态下的化学反应,从而使我们对纳米约束作用有了基本了解。
更新日期:2018-06-04
中文翻译:

等离子-纳米流体氢化装置对纳米材料分子的超材料增强红外光谱研究
纳米约束下的分子行为对于理解生物和纳米材料系统中的化学过程至关重要。我们在这里展示了一种红外光谱方法,通过使用等离子-纳米流体混合装置来表征被限制在几十个纳米腔中的分子的分子结构。该装置由金属纳米结构阵列和被纳米流体腔隔开的金属镜组成。它的配置可以将分子和光能限制为在物理化学上定义明确的纳米腔体内的局部表面等离激元。利用等离激元-分子耦合,可以以显着的灵敏度选择性地检测纳米约束分子的振动模式。用水作为概念验证样品,我们已经成功地测量了红外吸收特性,并阐明了封闭在10 nm腔中的水的分子结构。他们揭露了与散装水有关的强大的氢键网络。我们的方法还能够区分分子结构中的细微差别,从而揭示了承压水在数十纳米尺寸范围内的结垢行为。还发现这种影响不是由与接口的交互作用所驱动的。然而,受约束的几何形状本身会促进水的分子间相互作用,并导致氢键网络的改性。这项研究为 他们揭露了与散装水有关的强大的氢键网络。我们的方法还能够区分分子结构中的细微差别,从而揭示了承压水在数十纳米尺寸范围内的结垢行为。还发现这种影响不是由与接口的交互作用所驱动的。然而,受约束的几何形状本身会促进水的分子间相互作用,并导致氢键网络的改性。这项研究为 他们揭露了与散装水有关的强大的氢键网络。我们的方法还能够区分分子结构中的细微差别,从而揭示了承压水在数十纳米尺寸范围内的结垢行为。还发现这种影响不是由与接口的交互作用所驱动的。然而,受约束的几何形状本身会促进水的分子间相互作用,并导致氢键网络的改性。这项研究为 然而,受约束的几何形状本身会促进水的分子间相互作用,并导致氢键网络的改性。这项研究为 然而,受约束的几何形状本身会促进水的分子间相互作用,并导致氢键网络的改性。这项研究为原位探测纳米约束分子及其完整状态下的化学反应,从而使我们对纳米约束作用有了基本了解。









































 京公网安备 11010802027423号
京公网安备 11010802027423号