当前位置:
X-MOL 学术
›
Chem. Sci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Probing the interaction between the encapsulated water molecule and the fullerene cages in H2O@C60− and H2O@C59N−
Chemical Science ( IF 7.6 ) Pub Date : 2018-06-04 00:00:00 , DOI: 10.1039/c8sc01031e Guo-Zhu Zhu 1 , Yuan Liu 1 , Yoshifumi Hashikawa 2 , Qian-Fan Zhang 1 , Yasujiro Murata 2 , Lai-Sheng Wang 1
Chemical Science ( IF 7.6 ) Pub Date : 2018-06-04 00:00:00 , DOI: 10.1039/c8sc01031e Guo-Zhu Zhu 1 , Yuan Liu 1 , Yoshifumi Hashikawa 2 , Qian-Fan Zhang 1 , Yasujiro Murata 2 , Lai-Sheng Wang 1
Affiliation
|
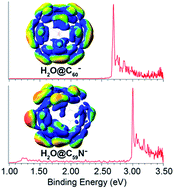
We report a high-resolution photoelectron imaging study of cryogenically-cooled H2O@C60− and H2O@C59N− endohedral fullerene anions. The electron affinity (EA) of H2O@C60 is measured to be 2.6923 ± 0.0008 eV, which is 0.0088 eV higher than the EA of C60, while the EA of H2O@C59N is measured to be 3.0058 eV ± 0.0007 eV, which is 0.0092 eV lower than the EA of C59N. The opposite shifts are found to be due to the different electrostatic interactions between the encapsulated water molecule and the fullerene cages in the two systems. There is a net coulombic attraction between the guest and host in H2O@C60−, but a repulsive interaction in H2O@C59N−. We have also observed low-frequency features in the photoelectron spectra tentatively attributed to the hindered rotational excitations of the encapsulated H2O molecule, providing further insights into the guest–host interactions in H2O@C60− and H2O@C59N−.
中文翻译:

探究 H2O@C60− 和 H2O@C59N− 中封装的水分子与富勒烯笼之间的相互作用
我们报告了低温冷却的 H 2 O@C 60 -和 H 2 O@C 59 N -内嵌富勒烯阴离子的高分辨率光电子成像研究。 H 2 O@C 60的电子亲和势(EA)为2.6923 ± 0.0008 eV,比C 60的EA高0.0088 eV,而H 2 O@C 59 N的EA为3.0058 eV ± 0.0007 eV,比 C 59 N 的 EA 低 0.0092 eV。发现相反的位移是由于两个系统中封装的水分子和富勒烯笼之间的不同静电相互作用所致。在H 2 O@C 60 −中,客体和主体之间存在净库仑吸引力,但在H 2 O@C 59 N −中存在排斥相互作用。 我们还观察到光电子能谱中的低频特征,初步归因于封装的 H 2 O 分子的受阻旋转激发,从而进一步深入了解 H 2 O@C 60 −和 H 2 O@C 中的客体-主体相互作用。 59 N - .
更新日期:2018-06-04
中文翻译:

探究 H2O@C60− 和 H2O@C59N− 中封装的水分子与富勒烯笼之间的相互作用
我们报告了低温冷却的 H 2 O@C 60 -和 H 2 O@C 59 N -内嵌富勒烯阴离子的高分辨率光电子成像研究。 H 2 O@C 60的电子亲和势(EA)为2.6923 ± 0.0008 eV,比C 60的EA高0.0088 eV,而H 2 O@C 59 N的EA为3.0058 eV ± 0.0007 eV,比 C 59 N 的 EA 低 0.0092 eV。发现相反的位移是由于两个系统中封装的水分子和富勒烯笼之间的不同静电相互作用所致。在H 2 O@C 60 −中,客体和主体之间存在净库仑吸引力,但在H 2 O@C 59 N −中存在排斥相互作用。 我们还观察到光电子能谱中的低频特征,初步归因于封装的 H 2 O 分子的受阻旋转激发,从而进一步深入了解 H 2 O@C 60 −和 H 2 O@C 中的客体-主体相互作用。 59 N - .











































 京公网安备 11010802027423号
京公网安备 11010802027423号