Acta Biomaterialia ( IF 9.4 ) Pub Date : 2018-06-03 , DOI: 10.1016/j.actbio.2018.05.050 Fengchun Tian , Fatima Zohra Dahmani , Jianan Qiao , Jiang Ni , Hui Xiong , Tengfei Liu , Jianping Zhou , Jing Yao
|
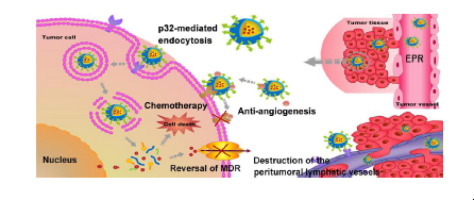
Several obstacles are currently impeding the successful treatment of breast cancer, namely impaired drug accumulation into the tumor site, toxicity to normal cells and narrow therapeutic index of chemotherapy, multidrug resistance (MDR) and the metastatic spread of cancer cells through the blood and lymphatic vessels. In this regard, we designed a novel multifunctional nano-sized drug delivery system based on LyP-1 peptide-modified low-molecular-weight heparin-quercetin conjugate (PLQ). This nanosystem was developed for targeted co-delivery of multiple anticancer drugs to p32-overexpressing tumor cells and peritumoral lymphatic vessels, using LyP-1 peptide as active targeting ligand, with the aim to achieve a targeted combinatorial chemo/angiostatic therapy and MDR reversal. The cellular uptake of PLQ nanoparticles by p32-overexpressing breast cancer cells was significantly higher than nonfunctionalized nanoparticles. Besides, the anti-angiogenic activity of PLQ nanoparticles was proven by the effective inhibition of the bFGF-induced neovascularization in subcutaneous Matrigel plugs. More importantly, PLQ/GA nanoparticles with better targeting ability toward p32-positive tumors, displayed a high antitumor outcome by inhibition of tumor cells proliferation and angiogenesis. Immunohistochemistry and western blot assay showed that PLQ/GA nanoparticles significantly disrupted the lymphatic formation of tumor, and inhibited the P-glycoprotein (P-gp) expression in MCF-7 tumor cells, respectively. In conclusion, PLQ/GA nanoparticles provide a synergistic strategy for effective targeted co-delivery of chemotherapeutic and antiangiogenic agents and reversing MDR and metastasis in breast cancer.
Statement of Significance
Herein, we successfully developed a novel amphiphilic nanomaterial, LyP-1-LMWH-Qu (PLQ) conjugate, consisting of a tumor-targeting moiety LyP-1, a hydrophobic quercetin (a multidrug resistance [MDR]–reversing drug) inner core, and a hydrophilic low-molecular-weight heparin (an antiangiogenic agent) outer shell for encapsulating and delivering a hydrophobic chemotherapeutic agent (gambogic acid). This versatile nanoplatform with multiple targeted features, i.e., dual chemo/angiostatic effects, destruction ability of the peritumoral lymphatic vessels, and reversal of MDR, resulted in a significantly stronger antitumor efficacy and lower toxic side effect than those of nontargeted nanoparticles and the free drug solution. Therefore, this versatile nanosystem might provide a novel insight for the treatment and palliation of breast cancer by targeted co-delivery of chemo/antiangiogenic agents and reversing MDR and metastasis.
中文翻译:

有针对性的纳米平台共同提供化疗药物和抗血管生成药物,作为逆转乳腺癌多药耐药性的工具
当前有几个障碍阻碍了乳腺癌的成功治疗,即,药物在肿瘤部位的蓄积受损,对正常细胞的毒性和化学疗法的狭窄治疗指数,多药耐药性(MDR)以及癌细胞通过血液和淋巴管的转移扩散。在这方面,我们设计了基于LyP-1肽修饰的低分子量肝素-槲皮素共轭物(PLQ)的新型多功能纳米药物递送系统。使用LyP-1肽作为活性靶向配体,开发了该纳米系统,用于将多种抗癌药物靶向共递送至过度表达p32的肿瘤细胞和肿瘤周围淋巴管,以实现靶向的组合化学/血管抑制疗法和MDR逆转。p32过表达的乳腺癌细胞对PLQ纳米颗粒的细胞摄取显着高于未功能化的纳米颗粒。此外,通过有效抑制皮下基质胶塞中bFGF诱导的新血管形成,证明了PLQ纳米颗粒具有抗血管生成活性。更重要的是,对p32阳性肿瘤具有更好的靶向能力的PLQ / GA纳米颗粒通过抑制肿瘤细胞的增殖和血管生成而表现出很高的抗肿瘤效果。免疫组织化学和western blot检测结果表明,PLQ / GA纳米颗粒显着破坏了肿瘤的淋巴形成,并抑制了MCF-7肿瘤细胞中P-糖蛋白(P-gp)的表达。综上所述,
重要声明
在这里,我们成功开发了一种新型的两亲性纳米材料LyP-1-LMWH-Qu(PLQ)共轭物,该共轭物由靶向肿瘤的部分LyP-1,疏水性槲皮素(一种多重耐药[MDR]逆转药物)的内芯组成,亲水性低分子量肝素(抗血管生成剂)外壳,用于包封和输送疏水性化学治疗剂(藤黄酸)。这种具有多种靶向功能的多功能纳米平台,具有双重化学/血管抑制作用,肿瘤周围淋巴管的破坏能力和MDR逆转,与非靶向纳米颗粒和游离药物相比,抗肿瘤功效显着增强,毒性副作用更低解决方案。所以,











































 京公网安备 11010802027423号
京公网安备 11010802027423号