当前位置:
X-MOL 学术
›
Angew. Chem. Int. Ed.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Oxidative C−H/C−H Cross‐Coupling Reactions between N‐Acylanilines and Benzamides Enabled by a Cp*‐Free RhCl3/TFA Catalytic System
Angewandte Chemie International Edition ( IF 16.1 ) Pub Date : 2018-06-19 , DOI: 10.1002/anie.201804528 Yang Shi 1 , Luoqiang Zhang 1 , Jingbo Lan 1 , Min Zhang 1 , Fulin Zhou 1 , Wenlong Wei 1 , Jingsong You 1
Angewandte Chemie International Edition ( IF 16.1 ) Pub Date : 2018-06-19 , DOI: 10.1002/anie.201804528 Yang Shi 1 , Luoqiang Zhang 1 , Jingbo Lan 1 , Min Zhang 1 , Fulin Zhou 1 , Wenlong Wei 1 , Jingsong You 1
Affiliation
|
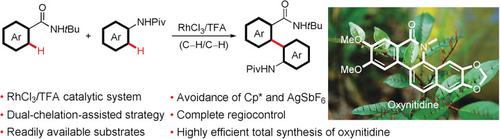
By making use of a dual‐chelation‐assisted strategy, a completely regiocontrolled oxidative C−H/C−H cross‐coupling reaction between an N‐acylaniline and a benzamide has been accomplished for the first time. This process constitutes a step‐economic and highly efficient pathway to 2‐amino‐2′‐carboxybiaryl scaffolds from readily available substrates. A Cp*‐free RhCl3/TFA catalytic system was developed to replace the [Cp*RhCl2]2/AgSbF6 system generally used in oxidative C−H/C−H cross‐coupling reactions between two (hetero)arenes (Cp*=pentamethylcyclopentadienyl, TFA=trifluoroacetic acid). The RhCl3/TFA system avoids the use of the expensive Cp* ligand and AgSbF6. As an illustrative example, the procedure developed herein greatly streamlines the total synthesis of the naturally occurring benzo[c]phenanthridine alkaloid oxynitidine, which was accomplished in excellent overall yield.
中文翻译:

无Cp *的RhCl3 / TFA催化系统在N-酰基苯胺和苯甲酰胺之间的氧化CH-H / C-H交叉偶联反应
通过使用双重螯合辅助策略,首次实现了N-丙氨酸与苯甲酰胺之间完全区域控制的氧化CH / C-H交叉偶联反应。此过程构成了从容易获得的底物制备2-氨基2'-羧基联芳基支架的经济高效的步骤。开发了无Cp *的RhCl 3 / TFA催化体系来代替通常用于两个(杂)芳烃(Cp)的氧化CH / CH交叉偶联反应中的[Cp * RhCl 2 ] 2 / AgSbF 6体系* =五甲基环戊二烯基,TFA =三氟乙酸)。RhCl 3 / TFA系统避免使用昂贵的Cp *配体和AgSbF 6。作为说明性实例,本文开发的方法极大地简化了天然存在的苯并[ c ]菲啶生物碱氧基亚硝胺的总合成,这以优异的总收率实现。
更新日期:2018-06-19
中文翻译:

无Cp *的RhCl3 / TFA催化系统在N-酰基苯胺和苯甲酰胺之间的氧化CH-H / C-H交叉偶联反应
通过使用双重螯合辅助策略,首次实现了N-丙氨酸与苯甲酰胺之间完全区域控制的氧化CH / C-H交叉偶联反应。此过程构成了从容易获得的底物制备2-氨基2'-羧基联芳基支架的经济高效的步骤。开发了无Cp *的RhCl 3 / TFA催化体系来代替通常用于两个(杂)芳烃(Cp)的氧化CH / CH交叉偶联反应中的[Cp * RhCl 2 ] 2 / AgSbF 6体系* =五甲基环戊二烯基,TFA =三氟乙酸)。RhCl 3 / TFA系统避免使用昂贵的Cp *配体和AgSbF 6。作为说明性实例,本文开发的方法极大地简化了天然存在的苯并[ c ]菲啶生物碱氧基亚硝胺的总合成,这以优异的总收率实现。









































 京公网安备 11010802027423号
京公网安备 11010802027423号