当前位置:
X-MOL 学术
›
Inorg. Chem. Front.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Novel hetero-bimetallic coordination polymer as a single source of highly dispersed Cu/Ni nanoparticles for efficient photocatalytic water splitting†
Inorganic Chemistry Frontiers ( IF 6.1 ) Pub Date : 2018-06-04 00:00:00 , DOI: 10.1039/c8qi00355f Shaista Ibrahim 1, 2, 3, 4, 5 , Imran Majeed 1, 2, 3, 4, 5 , Yuhong Qian 6, 7, 8 , Azhar Iqbal 1, 2, 3, 4, 5 , Dan Zhao 6, 7, 8 , David R. Turner 9, 10, 11, 12 , Muhammad Arif Nadeem 1, 2, 3, 4, 5
Inorganic Chemistry Frontiers ( IF 6.1 ) Pub Date : 2018-06-04 00:00:00 , DOI: 10.1039/c8qi00355f Shaista Ibrahim 1, 2, 3, 4, 5 , Imran Majeed 1, 2, 3, 4, 5 , Yuhong Qian 6, 7, 8 , Azhar Iqbal 1, 2, 3, 4, 5 , Dan Zhao 6, 7, 8 , David R. Turner 9, 10, 11, 12 , Muhammad Arif Nadeem 1, 2, 3, 4, 5
Affiliation
|
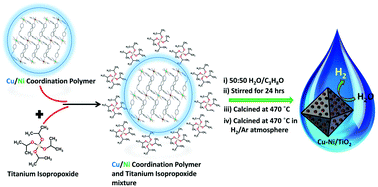
A new strategy for depositing highly dispersed Cu and Ni nanoparticles on the surface of TiO2 from a single source is demonstrated for photocatalytic hydrogen production. We used a newly synthesized cyanide-bridged hetero-bimetallic coordination polymer [{CuII(4,4′-dipy)2}{Ni(CN)4}]n·0.7(C2H6O2).1.6(H2O) (CP-1) (4,4′-dipy = 1,3-di(4-pyridyl)propane) as a single-source precursor of Cu–Ni nanoparticles. The structure of CP-1 was established by single-crystal X-ray diffraction analysis; CP-1 crystallizes in the monoclinic space group C2/c with β = 111.67(3)°. CP-1/TiO2 composites containing different weight percentages of CP-1 were achieved by hydrolyzing titanium isopropoxide in the presence of CP-1. Highly dispersed Cu and Ni nanoparticles were deposited on TiO2 by the calcination of the CP-1/TiO2 composites at different temperatures (420 °C, 470 °C and 520 °C) in air followed by reduction in H2/Ar atmosphere at 470 °C for 2 h. XRD, DRS/UV–Vis, TEM, Cu/Ni 2p XPS, and photoluminescence spectroscopy demonstrated the presence of CuO/Cu0 and NiO/Ni0 as active co-catalysts on the surface of TiO2. The 1 wt% Cu–Ni/TiO2-470 photocatalyst showed the maximum H2 production activity of 8.5 mmol h−1 g−1 in a glycerol/water mixture (20 vol%). The results are anticipated to direct the future development of efficient, low-cost and noble metal-free semiconductor photocatalysts for solar H2 production.
中文翻译:

新型异质双金属配位聚合物可作为单一来源的高度分散的Cu / Ni纳米粒子,可实现有效的光催化水分解†
展示了一种从单一来源在TiO 2表面上沉积高度分散的Cu和Ni纳米颗粒的新策略,用于光催化制氢。我们使用了新合成的氰化物桥联的异双金属配位聚合物[{Cu II(4,4'-dipy)2 } {Ni(CN)4 }] n ·0.7(C 2 H 6 O 2).1.6(H 2 O)(CP-1)(4,4'-dipy = 1,3-di(4-吡啶基)丙烷)作为Cu-Ni纳米粒子的单源前体。CP-1的结构通过单晶X射线衍射分析确定。CP-1结晶单斜空间群C ^ 2 / C ^与β = 111.67(3)°。通过在CP-1存在下水解异丙氧基钛,可以得到含有不同重量百分比CP-1的CP-1 / TiO 2复合材料。通过在不同温度(420°C,470°C和520°C)下在空气中煅烧CP-1 / TiO 2复合材料,然后在H 2 / Ar气氛中还原,将高度分散的Cu和Ni纳米颗粒沉积在TiO 2上在470°C下放置2小时。XRD,DRS / UV-Vis,TEM,Cu / Ni 2p XPS和光致发光光谱表明存在CuO / CuTiO 2表面上的活性助催化剂包括0和NiO / Ni 0。1%(重量)的Cu-Ni / TiO 2 -470光催化剂在甘油/水混合物(20%(体积))中显示出最大的H 2产生活性,为8.5 mmol h -1 g -1。预期结果将指导用于太阳能H 2生产的高效,低成本和无贵金属半导体光催化剂的未来发展。
更新日期:2018-06-04
中文翻译:

新型异质双金属配位聚合物可作为单一来源的高度分散的Cu / Ni纳米粒子,可实现有效的光催化水分解†
展示了一种从单一来源在TiO 2表面上沉积高度分散的Cu和Ni纳米颗粒的新策略,用于光催化制氢。我们使用了新合成的氰化物桥联的异双金属配位聚合物[{Cu II(4,4'-dipy)2 } {Ni(CN)4 }] n ·0.7(C 2 H 6 O 2).1.6(H 2 O)(CP-1)(4,4'-dipy = 1,3-di(4-吡啶基)丙烷)作为Cu-Ni纳米粒子的单源前体。CP-1的结构通过单晶X射线衍射分析确定。CP-1结晶单斜空间群C ^ 2 / C ^与β = 111.67(3)°。通过在CP-1存在下水解异丙氧基钛,可以得到含有不同重量百分比CP-1的CP-1 / TiO 2复合材料。通过在不同温度(420°C,470°C和520°C)下在空气中煅烧CP-1 / TiO 2复合材料,然后在H 2 / Ar气氛中还原,将高度分散的Cu和Ni纳米颗粒沉积在TiO 2上在470°C下放置2小时。XRD,DRS / UV-Vis,TEM,Cu / Ni 2p XPS和光致发光光谱表明存在CuO / CuTiO 2表面上的活性助催化剂包括0和NiO / Ni 0。1%(重量)的Cu-Ni / TiO 2 -470光催化剂在甘油/水混合物(20%(体积))中显示出最大的H 2产生活性,为8.5 mmol h -1 g -1。预期结果将指导用于太阳能H 2生产的高效,低成本和无贵金属半导体光催化剂的未来发展。











































 京公网安备 11010802027423号
京公网安备 11010802027423号