当前位置:
X-MOL 学术
›
Bioconjugate Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
The Effect of Lipidation on the Self-Assembly of the Gut-Derived Peptide Hormone PYY3–36
Bioconjugate Chemistry ( IF 4.0 ) Pub Date : 2018-06-01 00:00:00 , DOI: 10.1021/acs.bioconjchem.8b00286 Jessica A. Hutchinson 1 , Samuel Burholt 1 , Ian W. Hamley 1 , Anna-Karin Lundback 2 , Shahid Uddin 2 , Ana Gomes dos Santos 2 , Mehedi Reza 3 , Jani Seitsonen 3 , Janne Ruokolainen 3
Bioconjugate Chemistry ( IF 4.0 ) Pub Date : 2018-06-01 00:00:00 , DOI: 10.1021/acs.bioconjchem.8b00286 Jessica A. Hutchinson 1 , Samuel Burholt 1 , Ian W. Hamley 1 , Anna-Karin Lundback 2 , Shahid Uddin 2 , Ana Gomes dos Santos 2 , Mehedi Reza 3 , Jani Seitsonen 3 , Janne Ruokolainen 3
Affiliation
|
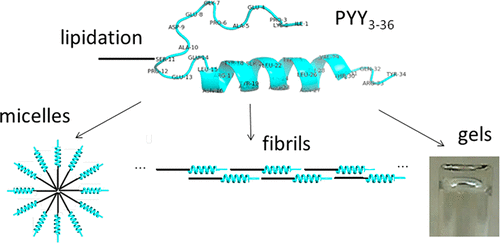
Lipidation is a powerful strategy to improve the stability in vivo of peptide drugs. Attachment of a lipid chain to a hydrophilic peptide leads to amphiphilicity and the potential for surfactant-like self-assembly. Here, the self-assembly and conformation of three lipidated derivatives of the gastrointestinal peptide hormone PYY3–36 is examined using a comprehensive range of spectroscopic, scattering, and electron microscopy methods and compared to those of the parent PYY3–36 peptide. The peptides are lipidated at Ser(11), Arg(17), or Arg(23) in the peptide; the former is within the β-turn domain (based on the published solution NMR structure), and the latter two are both within the α-helical domain. We show that it is possible to access a remarkable diversity of nanostructures ranging from micelles to nanotapes and fibrillar hydrogels by control of assembly conditions (concentration, pH, and temperature). All of the lipopeptides self-assemble above a critical aggregation concentration (cac), determined through pyrene fluorescence probe measurements, and they all have predominantly α-helical secondary structure at their native pH. The pH and temperature dependence of the α-helical conformation were probed via circular dichroism spectroscopy experiments. Lipidation was found to provide enhanced stability against changes in temperature and pH. The self-assembled structures were investigated using small-angle X-ray scattering (SAXS) and cryogenic transmission electron microscopy (cryo-TEM). Distinct differences in nanostructure were observed for lipidated and unlipidated peptides, also depending on the position of lipidation. Remarkably, micelles containing lipopeptides with α-helical peptide conformation were observed. Gelation was observed at higher concentrations in certain pH intervals for the lipidated peptides, but not for unlipidated PYY3–36. Thus, lipidation, in addition to enhancing stability against pH and temperature variation, also provides a route to prepare PYY peptide hydrogels. These findings provide important insights into the control of PYY3–36 conformation and aggregation by lipidation, relevant to the development of future therapeutics based on this peptide hormone, for example, in treatments for obesity.
中文翻译:

脂化对肠道衍生肽激素PYY 3–36自组装的影响
脂质化是提高肽药物体内稳定性的有效策略。脂质链与亲水性肽的连接会导致两亲性,并可能产生类似表面活性剂的自组装。在这里,使用一系列光谱,散射和电子显微镜方法检查了胃肠肽激素PYY 3–36的三种脂化衍生物的自组装和构象,并将其与母体PYY 3–36进行了比较。肽。肽在肽的Ser(11),Arg(17)或Arg(23)处脂化;前者在β-turn域内(基于已发布的溶液NMR结构),而后两者均在α-螺旋域内。我们表明,可以通过控制组装条件(浓度,pH和温度)来访问从胶束到纳米带和原纤维水凝胶的大量纳米结构。通过pyr荧光探针测量确定,所有脂肽都在临界聚集浓度(cac)以上自组装,它们在其天然pH值上均主要具有α-螺旋二级结构。通过圆二色光谱实验探究了α-螺旋构象的pH和温度依赖性。发现脂质作用提供了针对温度和pH变化的增强的稳定性。使用小角度X射线散射(SAXS)和低温透射电子显微镜(cryo-TEM)研究了自组装结构。脂化和未脂化的肽在纳米结构上观察到不同的差异,这也取决于脂化的位置。明显地,观察到含有具有α-螺旋肽构象的脂肽的胶束。在一定的pH间隔内,脂化肽在较高浓度下观察到凝胶化,而未脂化的PYY没有观察到胶凝 脂化和未脂化的肽在纳米结构上观察到不同的差异,这也取决于脂化的位置。明显地,观察到含有具有α-螺旋肽构象的脂肽的胶束。在一定的pH间隔内,脂化肽在较高浓度下观察到凝胶化,而未脂化的PYY没有观察到胶凝 脂化和未脂化的肽在纳米结构上观察到不同的差异,这也取决于脂化的位置。明显地,观察到含有具有α-螺旋肽构象的脂肽的胶束。在一定的pH间隔内,脂化肽在较高浓度下观察到凝胶化,而未脂化的PYY没有观察到胶凝3–36。因此,脂质化除了增强针对pH和温度变化的稳定性之外,还提供了制备PYY肽水凝胶的途径。这些发现为通过脂化控制PYY 3–36构象和聚集提供了重要的见识,这与基于这种肽激素的未来疗法的开发有关,例如在肥胖症治疗中。
更新日期:2018-06-01
中文翻译:

脂化对肠道衍生肽激素PYY 3–36自组装的影响
脂质化是提高肽药物体内稳定性的有效策略。脂质链与亲水性肽的连接会导致两亲性,并可能产生类似表面活性剂的自组装。在这里,使用一系列光谱,散射和电子显微镜方法检查了胃肠肽激素PYY 3–36的三种脂化衍生物的自组装和构象,并将其与母体PYY 3–36进行了比较。肽。肽在肽的Ser(11),Arg(17)或Arg(23)处脂化;前者在β-turn域内(基于已发布的溶液NMR结构),而后两者均在α-螺旋域内。我们表明,可以通过控制组装条件(浓度,pH和温度)来访问从胶束到纳米带和原纤维水凝胶的大量纳米结构。通过pyr荧光探针测量确定,所有脂肽都在临界聚集浓度(cac)以上自组装,它们在其天然pH值上均主要具有α-螺旋二级结构。通过圆二色光谱实验探究了α-螺旋构象的pH和温度依赖性。发现脂质作用提供了针对温度和pH变化的增强的稳定性。使用小角度X射线散射(SAXS)和低温透射电子显微镜(cryo-TEM)研究了自组装结构。脂化和未脂化的肽在纳米结构上观察到不同的差异,这也取决于脂化的位置。明显地,观察到含有具有α-螺旋肽构象的脂肽的胶束。在一定的pH间隔内,脂化肽在较高浓度下观察到凝胶化,而未脂化的PYY没有观察到胶凝 脂化和未脂化的肽在纳米结构上观察到不同的差异,这也取决于脂化的位置。明显地,观察到含有具有α-螺旋肽构象的脂肽的胶束。在一定的pH间隔内,脂化肽在较高浓度下观察到凝胶化,而未脂化的PYY没有观察到胶凝 脂化和未脂化的肽在纳米结构上观察到不同的差异,这也取决于脂化的位置。明显地,观察到含有具有α-螺旋肽构象的脂肽的胶束。在一定的pH间隔内,脂化肽在较高浓度下观察到凝胶化,而未脂化的PYY没有观察到胶凝3–36。因此,脂质化除了增强针对pH和温度变化的稳定性之外,还提供了制备PYY肽水凝胶的途径。这些发现为通过脂化控制PYY 3–36构象和聚集提供了重要的见识,这与基于这种肽激素的未来疗法的开发有关,例如在肥胖症治疗中。











































 京公网安备 11010802027423号
京公网安备 11010802027423号