Bioorganic Chemistry ( IF 4.5 ) Pub Date : 2018-06-02 , DOI: 10.1016/j.bioorg.2018.06.001 Mohammed Gollapalli , Muhammad Taha , Hayat Ullah , Muhammad Nawaz , Laode Muhammad Ramadhan AlMuqarrabun , Fazal Rahim , Faiza Qureshi , Ashik Mosaddik , Norizan Ahmat , Khalid Mohammed Khan
|
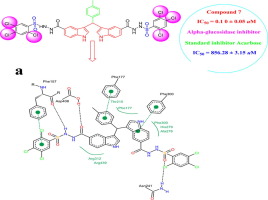
In search of better α-glucosidase inhibitors, a series of bis-indolylmethane sulfonohydrazides derivatives (1-14) were synthesized and evaluated for their α-glucosidase inhibitory potential. All derivatives exhibited outstanding α-glucosidase inhibition with IC50 values ranging between 0.10 ± 0.05 to 5.1 ± 0.05 μM when compared with standard drug acarbose having IC50 value 856.28 ± 3.15 μM. Among the series, analog 7 (0.10 ± 0.05 μM) with tri-chloro substitution on phenyl ring was identified as the most potent inhibitor of α-glucosidase (∼ 8500 times). The structure activity relationship has been also established. Molecular docking studies were also performed to help understand the binding interaction of the most active analogs with receptors. From the docking studies, it was observed that all the active bis-indolylmethane sulfonohydrazides derivatives showed considerable binding interactions within the active site (acarbose inhibition site) of α-glucosidase. We also evaluated toxicity of all derivatives and found none of them are toxic.
中文翻译:

作为有效的α-葡萄糖苷酶抑制剂的双吲哚甲烷磺酰肼衍生物的合成
在寻找更好的α葡糖苷酶抑制剂,一系列的双-indolylmethane sulfonohydrazides衍生物(1 - 14)的合成和评价它们的α葡萄糖苷酶抑制潜力。与标准药物阿卡波糖的IC 50值为856.28±3.15μM相比,所有衍生物均表现出出色的α-葡萄糖苷酶抑制作用,IC 50值为0.10±0.05至5.1±0.05μM 。在该系列中,苯环上被三氯取代的类似物7(0.10±0.05μM)被确定为最有效的α抑制剂。-葡糖苷酶(〜8500次)。结构活动关系也已建立。还进行了分子对接研究,以帮助了解最活跃的类似物与受体的结合相互作用。从对接研究中,观察到所有的活性双吲哚基甲烷磺酰肼衍生物在α-葡糖苷酶的活性位点(阿卡波糖抑制位点)内显示出相当大的结合相互作用。我们还评估了所有衍生物的毒性,发现它们均无毒。











































 京公网安备 11010802027423号
京公网安备 11010802027423号