当前位置:
X-MOL 学术
›
Org. Process Res. Dev.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Practical Synthesis of a 6-Triazolylazabicyclo[3.1.0]hexane
Organic Process Research & Development ( IF 3.1 ) Pub Date : 2018-06-01 00:00:00 , DOI: 10.1021/acs.oprd.8b00101 Lauren E. Sirois 1 , Jie Xu 1 , Remy Angelaud 1 , David Lao 1 , Francis Gosselin 1
Organic Process Research & Development ( IF 3.1 ) Pub Date : 2018-06-01 00:00:00 , DOI: 10.1021/acs.oprd.8b00101 Lauren E. Sirois 1 , Jie Xu 1 , Remy Angelaud 1 , David Lao 1 , Francis Gosselin 1
Affiliation
|
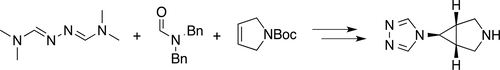
We describe a practical, scalable synthesis of an advanced heterocyclic intermediate, (1R,5S,6s)-6-(4H-1,2,4-triazol-4-yl)-3-azabicyclo[3.1.0]hexane. A robust synthetic sequence based on a Kulinkovich–de Meijere pyrroline cyclopropanation followed by transamination of N,N′-dimethylformamide azine with the resultant amine was developed to supply >18 kg of the target triazolyl azabicycle with 98 wt % purity in its free base form. Reaction conditions and isolation methods for the key 1,2,4-triazole formation step were explored to minimize undesired reaction pathways and to increase the purity of the product. Additionally, at several stages different freebasing methods were implemented that addressed the high water solubility of the associated nitrogen-rich compounds.
中文翻译:

实际合成6-三唑基氮杂双环[3.1.0]己烷
我们描述了先进的杂环中间体,(1 R,5 S,6 s)-6-(4 H -1,2,4-三唑-4-基)-3-氮杂双环[3.1.0]的实用,可扩展的合成正己烷。基于Kulinkovich-de Meijere吡咯啉环丙烷化反应的稳健合成序列,随后进行N,N的氨基转移开发了具有所得胺的'-二甲基甲酰胺嗪,以其游离碱形式提供> 18 kg纯度为98 wt%的目标三唑基氮杂双环。探索了关键的1,2,4-三唑形成步骤的反应条件和分离方法,以最大程度地减少不良反应途径并提高产物的纯度。另外,在几个阶段实施了不同的游离碱法,以解决相关富氮化合物的高水溶性。
更新日期:2018-06-01
中文翻译:

实际合成6-三唑基氮杂双环[3.1.0]己烷
我们描述了先进的杂环中间体,(1 R,5 S,6 s)-6-(4 H -1,2,4-三唑-4-基)-3-氮杂双环[3.1.0]的实用,可扩展的合成正己烷。基于Kulinkovich-de Meijere吡咯啉环丙烷化反应的稳健合成序列,随后进行N,N的氨基转移开发了具有所得胺的'-二甲基甲酰胺嗪,以其游离碱形式提供> 18 kg纯度为98 wt%的目标三唑基氮杂双环。探索了关键的1,2,4-三唑形成步骤的反应条件和分离方法,以最大程度地减少不良反应途径并提高产物的纯度。另外,在几个阶段实施了不同的游离碱法,以解决相关富氮化合物的高水溶性。











































 京公网安备 11010802027423号
京公网安备 11010802027423号