当前位置:
X-MOL 学术
›
Chem. Sci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Radical difluoromethylthiolation of aromatics enabled by visible light†
Chemical Science ( IF 7.6 ) Pub Date : 2018-06-01 00:00:00 , DOI: 10.1039/c8sc01669k Jianbin Li 1, 2, 3, 4 , Dianhu Zhu 1, 2, 3, 4 , Leiyang Lv 1, 2, 3, 4 , Chao-Jun Li 1, 2, 3, 4
Chemical Science ( IF 7.6 ) Pub Date : 2018-06-01 00:00:00 , DOI: 10.1039/c8sc01669k Jianbin Li 1, 2, 3, 4 , Dianhu Zhu 1, 2, 3, 4 , Leiyang Lv 1, 2, 3, 4 , Chao-Jun Li 1, 2, 3, 4
Affiliation
|
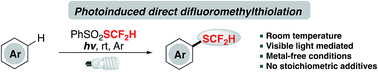
Direct introduction of a difluoromethylthio group (–SCF2H) to arenes represents an efficient route to access a valuable catalogue of organofluorines; however, to realize this transformation under metal-free and mild conditions still remains challenging and rarely reported. Herein, a metal-catalyst-free and redox-neutral innate difluoromethylthiolation method with a shelf-stable and readily available reagent, PhSO2SCF2H, under visible light irradiation is described. This light-mediated protocol successfully converts a broad spectrum of arenes and heteroarenes to difluoromethylthioethers in the absence of noble metals and stoichiometric amounts of additives.
中文翻译:

可见光可实现芳烃的自由基二氟甲基硫醇化†
在芳烃中直接引入二氟甲硫基(–SCF 2 H)是获得有价值的有机氟目录的有效途径;然而,在无金属和温和条件下实现这种转变仍然具有挑战性,并且鲜有报道。本文中,描述了在可见光照射下使用货架稳定且容易获得的试剂PhSO 2 SCF 2 H的无金属催化剂和氧化还原中性的固有二氟甲基硫醇化方法。在没有贵金属和化学计量的添加剂的情况下,这种光介导的方案成功地将广谱的芳烃和杂芳烃转化为二氟甲基硫醚。
更新日期:2018-06-01
中文翻译:

可见光可实现芳烃的自由基二氟甲基硫醇化†
在芳烃中直接引入二氟甲硫基(–SCF 2 H)是获得有价值的有机氟目录的有效途径;然而,在无金属和温和条件下实现这种转变仍然具有挑战性,并且鲜有报道。本文中,描述了在可见光照射下使用货架稳定且容易获得的试剂PhSO 2 SCF 2 H的无金属催化剂和氧化还原中性的固有二氟甲基硫醇化方法。在没有贵金属和化学计量的添加剂的情况下,这种光介导的方案成功地将广谱的芳烃和杂芳烃转化为二氟甲基硫醚。











































 京公网安备 11010802027423号
京公网安备 11010802027423号