当前位置:
X-MOL 学术
›
Biomacromolecules
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Photoswitching of Cell Penetration of Amphipathic Peptides by Control of α-Helical Conformation
Biomacromolecules ( IF 5.5 ) Pub Date : 2018-06-01 00:00:00 , DOI: 10.1021/acs.biomac.8b00428 Gyu Chan Kim , Joon Hyung Ahn , Jae Hoon Oh , Sohee Nam , Soonsil Hyun , Jaehoon Yu , Yan Lee
Biomacromolecules ( IF 5.5 ) Pub Date : 2018-06-01 00:00:00 , DOI: 10.1021/acs.biomac.8b00428 Gyu Chan Kim , Joon Hyung Ahn , Jae Hoon Oh , Sohee Nam , Soonsil Hyun , Jaehoon Yu , Yan Lee
|
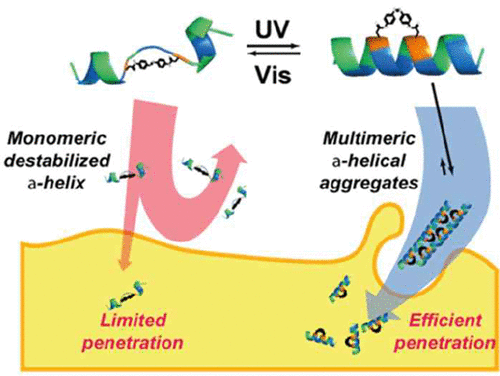
We stapled an amphipathic peptide mainly consisting of leucine (L) and lysine (K) by an azobenzene (Ab) linker for photocontrol of the secondary structure. The cis–trans isomerization of the Ab moieties could stabilize and destabilize the α-helical conformation of the LK peptide along with dramatic change of associated peptide structures in a reversible manner by UV–vis irradiation. The cell-penetrating activities of the LK peptide can be readily regulated by the photocontrol, as the stabilized cis-Ab-LK peptide showed remarkable increase of cell penetration compared to the destabilized trans-Ab-LK peptide. The photoswitchable cell-penetrating peptides would provide important structural information for cell permeability as well as an effective targeting strategy for peptide-based pharmaceuticals with spatiotemporal specificity.
中文翻译:

通过控制α-螺旋构象对两亲性肽的细胞渗透进行光开关。
我们通过偶氮苯(Ab)接头装订了主要由亮氨酸(L)和赖氨酸(K)组成的两亲性肽,用于对二级结构进行光控。Ab部分的顺式-反式异构化可以稳定和破坏LK肽的α螺旋构象以及相关肽结构的显着变化(通过UV-vis辐射可逆)。LK肽的细胞穿透活性可以很容易地通过光控调节,因为稳定的顺式-Ab-LK肽与不稳定的反式相比显示出显着的细胞渗透性增加-Ab-LK肽。光可穿透细胞的肽将为细胞的通透性提供重要的结构信息,并为具有时空特异性的基于肽的药物提供有效的靶向策略。
更新日期:2018-06-01
中文翻译:

通过控制α-螺旋构象对两亲性肽的细胞渗透进行光开关。
我们通过偶氮苯(Ab)接头装订了主要由亮氨酸(L)和赖氨酸(K)组成的两亲性肽,用于对二级结构进行光控。Ab部分的顺式-反式异构化可以稳定和破坏LK肽的α螺旋构象以及相关肽结构的显着变化(通过UV-vis辐射可逆)。LK肽的细胞穿透活性可以很容易地通过光控调节,因为稳定的顺式-Ab-LK肽与不稳定的反式相比显示出显着的细胞渗透性增加-Ab-LK肽。光可穿透细胞的肽将为细胞的通透性提供重要的结构信息,并为具有时空特异性的基于肽的药物提供有效的靶向策略。











































 京公网安备 11010802027423号
京公网安备 11010802027423号