当前位置:
X-MOL 学术
›
ChemBioChem
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Unravelling the Specificity of Laminaribiose Phosphorylase from Paenibacillus sp. YM-1 towards Donor Substrates Glucose/Mannose 1-Phosphate by Using X-ray Crystallography and Saturation Transfer Difference NMR Spectroscopy.
ChemBioChem ( IF 2.6 ) Pub Date : 2018-07-04 , DOI: 10.1002/cbic.201800260 Sakonwan Kuhaudomlarp 1 , Samuel Walpole 2 , Clare E M Stevenson 1 , Sergey A Nepogodiev 1 , David M Lawson 1 , Jesus Angulo 2 , Robert A Field 1
ChemBioChem ( IF 2.6 ) Pub Date : 2018-07-04 , DOI: 10.1002/cbic.201800260 Sakonwan Kuhaudomlarp 1 , Samuel Walpole 2 , Clare E M Stevenson 1 , Sergey A Nepogodiev 1 , David M Lawson 1 , Jesus Angulo 2 , Robert A Field 1
Affiliation
|
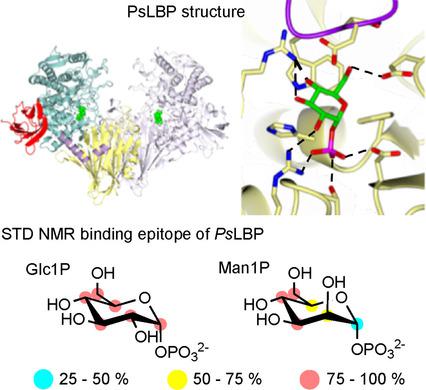
Glycoside phosphorylases (GPs) carry out a reversible phosphorolysis of carbohydrates into oligosaccharide acceptors and the corresponding sugar 1-phosphates. The reversibility of the reaction enables the use of GPs as biocatalysts for carbohydrate synthesis. Glycosyl hydrolase family 94 (GH94), which only comprises GPs, is one of the most studied GP families that have been used as biocatalysts for carbohydrate synthesis, in academic research and in industrial production. Understanding the mechanism of GH94 enzymes is a crucial step towards enzyme engineering to improve and expand the applications of these enzymes in synthesis. In this work with a GH94 laminaribiose phosphorylase from Paenibacillus sp. YM-1 (PsLBP), we have demonstrated an enzymatic synthesis of disaccharide 1 (β-d-mannopyranosyl-(1→3)-d-glucopyranose) by using a natural acceptor glucose and noncognate donor substrate α-mannose 1-phosphate (Man1P). To investigate how the enzyme recognises different sugar 1-phosphates, the X-ray crystal structures of PsLBP in complex with Glc1P and Man1P have been solved, providing the first molecular detail of the recognition of a noncognate donor substrate by GPs, which revealed the importance of hydrogen bonding between the active site residues and hydroxy groups at C2, C4, and C6 of sugar 1-phosphates. Furthermore, we used saturation transfer difference NMR spectroscopy to support crystallographic studies on the sugar 1-phosphates, as well as to provide further insights into the PsLBP recognition of the acceptors and disaccharide products.
中文翻译:

从类芽孢杆菌中揭示海带二糖磷酸化酶的特异性。YM-1 对供体底物葡萄糖/甘露糖 1-磷酸盐的使用 X 射线晶体学和饱和转移差 NMR 光谱。
糖苷磷酸化酶 (GP) 将碳水化合物可逆地磷酸化为寡糖受体和相应的糖 1-磷酸盐。该反应的可逆性使得 GPs 能够用作碳水化合物合成的生物催化剂。仅包含 GP 的糖基水解酶家族 94 (GH94) 是研究最多的 GP 家族之一,已在学术研究和工业生产中用作碳水化合物合成的生物催化剂。了解 GH94 酶的作用机制是酶工程改进和扩大这些酶在合成中的应用的关键一步。在这项工作中,使用来自 Paenibacillus sp. 的 GH94 海带二糖磷酸化酶。YM-1 (PsLBP), 我们已经证明了通过使用天然受体葡萄糖和非同源供体底物 α-甘露糖 1-磷酸 (Man1P) 酶促合成二糖 1 (β-d-吡喃甘露糖基-(1→3)-d-吡喃葡萄糖)。为了研究该酶如何识别不同的糖 1-磷酸,已经解决了与 Glc1P 和 Man1P 复合的 PsLBP 的 X 射线晶体结构,提供了 GP 识别非同源供体底物的第一个分子细节,这揭示了重要性糖 1-磷酸的 C2、C4 和 C6 处的活性位点残基和羟基之间的氢键。此外,我们使用饱和转移差异核磁共振光谱来支持对糖 1-磷酸盐的晶体学研究,并提供对受体和二糖产物的 PsLBP 识别的进一步见解。
更新日期:2018-07-04
中文翻译:

从类芽孢杆菌中揭示海带二糖磷酸化酶的特异性。YM-1 对供体底物葡萄糖/甘露糖 1-磷酸盐的使用 X 射线晶体学和饱和转移差 NMR 光谱。
糖苷磷酸化酶 (GP) 将碳水化合物可逆地磷酸化为寡糖受体和相应的糖 1-磷酸盐。该反应的可逆性使得 GPs 能够用作碳水化合物合成的生物催化剂。仅包含 GP 的糖基水解酶家族 94 (GH94) 是研究最多的 GP 家族之一,已在学术研究和工业生产中用作碳水化合物合成的生物催化剂。了解 GH94 酶的作用机制是酶工程改进和扩大这些酶在合成中的应用的关键一步。在这项工作中,使用来自 Paenibacillus sp. 的 GH94 海带二糖磷酸化酶。YM-1 (PsLBP), 我们已经证明了通过使用天然受体葡萄糖和非同源供体底物 α-甘露糖 1-磷酸 (Man1P) 酶促合成二糖 1 (β-d-吡喃甘露糖基-(1→3)-d-吡喃葡萄糖)。为了研究该酶如何识别不同的糖 1-磷酸,已经解决了与 Glc1P 和 Man1P 复合的 PsLBP 的 X 射线晶体结构,提供了 GP 识别非同源供体底物的第一个分子细节,这揭示了重要性糖 1-磷酸的 C2、C4 和 C6 处的活性位点残基和羟基之间的氢键。此外,我们使用饱和转移差异核磁共振光谱来支持对糖 1-磷酸盐的晶体学研究,并提供对受体和二糖产物的 PsLBP 识别的进一步见解。











































 京公网安备 11010802027423号
京公网安备 11010802027423号