当前位置:
X-MOL 学术
›
J. Phys. Chem. Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Ethylene Carbonate-Based Electrolyte Decomposition and Solid–Electrolyte Interphase Formation on Ca Metal Anodes
The Journal of Physical Chemistry Letters ( IF 4.8 ) Pub Date : 2018-06-01 00:00:00 , DOI: 10.1021/acs.jpclett.8b01261 Joshua Young 1 , Manuel Smeu 1
The Journal of Physical Chemistry Letters ( IF 4.8 ) Pub Date : 2018-06-01 00:00:00 , DOI: 10.1021/acs.jpclett.8b01261 Joshua Young 1 , Manuel Smeu 1
Affiliation
|
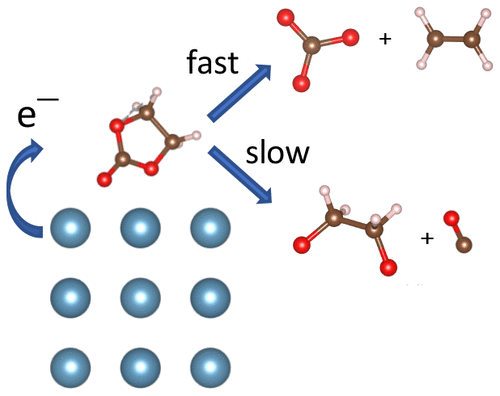
The formation of a solid–electrolyte interphase (SEI) in multivalent ion batteries, resulting from the decomposition of organic solvents at the anode interface, is a major bottleneck to their development as it prevents ionic diffusion and reversible stripping and plating. To gain insight into SEI formation in these systems, we investigate the decomposition of pure ethylene carbonate (EC) and an EC/Ca(ClO4)2 electrolyte on a Ca metal surface using density functional theory and ab initio molecular dynamics calculations. We first find that CaCO3, CaO, and Ca(OH)2 are all primary inorganic SEI components. We then investigate the reaction mechanisms of this decomposition, finding that although a fast two-electron reduction producing CO32– and C2H4 is thermodynamically and kinetically favorable, a reaction producing C2H4O22– and CO dominates when multiple EC molecules are considered. Finally, we find similar results upon the inclusion of Ca(ClO4)2 salt.
中文翻译:

Ca金属阳极上碳酸亚乙酯基电解质的分解和固电解质间相的形成
由于阳极界面上有机溶剂的分解,在多价离子电池中形成固体-电解质中间相(SEI)是其发展的主要瓶颈,因为它可以防止离子扩散以及可逆的剥离和镀覆。为了深入了解这些系统中的SEI形成,我们使用密度泛函理论和从头算分子动力学计算方法研究了Ca金属表面上纯碳酸亚乙酯(EC)和EC / Ca(ClO 4)2电解质的分解。我们首先发现CaCO 3,CaO和Ca(OH)2都是主要的无机SEI成分。然后我们研究了这种分解的反应机理,发现尽管快速产生两个电子的还原反应产生CO 3 2–和C 2 H 4在热力学和动力学上都是有利的,但当反应发生时,产生C 2 H 4 O 2 2–和CO的反应占主导地位。考虑多个EC分子。最后,在加入Ca(ClO 4)2盐后,我们发现了相似的结果。
更新日期:2018-06-01
中文翻译:

Ca金属阳极上碳酸亚乙酯基电解质的分解和固电解质间相的形成
由于阳极界面上有机溶剂的分解,在多价离子电池中形成固体-电解质中间相(SEI)是其发展的主要瓶颈,因为它可以防止离子扩散以及可逆的剥离和镀覆。为了深入了解这些系统中的SEI形成,我们使用密度泛函理论和从头算分子动力学计算方法研究了Ca金属表面上纯碳酸亚乙酯(EC)和EC / Ca(ClO 4)2电解质的分解。我们首先发现CaCO 3,CaO和Ca(OH)2都是主要的无机SEI成分。然后我们研究了这种分解的反应机理,发现尽管快速产生两个电子的还原反应产生CO 3 2–和C 2 H 4在热力学和动力学上都是有利的,但当反应发生时,产生C 2 H 4 O 2 2–和CO的反应占主导地位。考虑多个EC分子。最后,在加入Ca(ClO 4)2盐后,我们发现了相似的结果。











































 京公网安备 11010802027423号
京公网安备 11010802027423号