当前位置:
X-MOL 学术
›
J. Phys. Chem. B
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Single-Molecule Spectroscopy Study of Crowding-Induced Protein Spontaneous Denature and Crowding-Perturbed Unfolding–Folding Conformational Fluctuation Dynamics
The Journal of Physical Chemistry B ( IF 2.8 ) Pub Date : 2018-06-25 , DOI: 10.1021/acs.jpcb.8b03119 Zijiang Wang 1 , H. Peter Lu 1
The Journal of Physical Chemistry B ( IF 2.8 ) Pub Date : 2018-06-25 , DOI: 10.1021/acs.jpcb.8b03119 Zijiang Wang 1 , H. Peter Lu 1
Affiliation
|
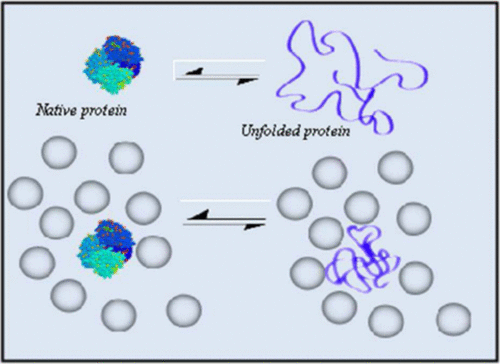
The effects of molecular crowding on protein folding–unfolding processes are of importance for understanding protein function and structure dynamics in living cells. The enhancement of protein stability as a result of reduced entropic effect in the presence of molecular crowding is well understood both experimentally and theoretically. However, because of the complexity and interplay between various interactions existing in an equally favored environment of protein folding and unfolding conformational dynamics, such a simple reduced entropic enhancement model does not suffice to describe protein folding conformational dynamics under a protein crowding condition. In this paper, we report our observation on that single protein molecules spontaneously denature into unfolded proteins and folding–unfolding fluctuations in solution of crowding reagent Ficoll 70. We have identified that such protein dynamics involves a combined mechanism of polymer–polymer interaction, entropic effects, and protein solvation dynamics. We characterize the protein folding–unfolding dynamics by using single-molecule spectroscopy to obtain detailed molecular dynamic scale information on the protein folding–unfolding conformational fluctuation dynamics. Our findings suggest that the complex unfolding dynamic processes are spontaneous denature of single protein molecules induced by molecular crowding effect which has been elusive for analysis in ensemble-averaged measurements. Furthermore, the energy needed for the spontaneous unfolding is at the biological accessible force fluctuation level, which suggests a strong implication of significant human health relevance and importance. The new knowledge of the inhomogeneous protein unfolding processes can serve as a step forward to a mechanistic understanding of human diseases associated with molecular crowding, protein aggregates, fibril formation, as well as gene translational regulation processes typically under a molecular crowded local environment.
中文翻译:

拥挤诱导的蛋白质自发变性和拥挤扰动的展开-折叠构象涨落动力学的单分子光谱研究
分子拥挤对蛋白质折叠-展开过程的影响对于了解活细胞中蛋白质的功能和结构动力学非常重要。在分子拥挤的情况下,由于熵作用降低而导致的蛋白质稳定性增强在实验和理论上都是众所周知的。然而,由于在蛋白质折叠和展开构象动力学的同样有利的环境中存在的各种相互作用之间的复杂性和相互作用,因此这种简单的减少的熵增强模型不足以描述蛋白质拥挤条件下的蛋白质折叠构象动力学。在本文中,我们报告了关于单个蛋白质分子自发地变性为未折叠蛋白质以及在拥挤试剂Ficoll 70溶液中折叠-展开的波动的观察结果。我们已经确定,这种蛋白质动力学涉及聚合物-聚合物相互作用,熵效应和蛋白质溶剂化的联合机制。动力学。我们通过使用单分子光谱学来表征蛋白质折叠-展开动力学,以获取有关蛋白质折叠-展开构象动力学的详细分子动力学尺度信息。我们的发现表明,复杂的展开动态过程是由分子拥挤效应诱导的单个蛋白质分子的自发变性,这在整体平均测量中难以捉摸。此外,自发展开所需的能量处于生物可及的力量波动水平,这暗示着人类健康的重要性和重要性。不均一的蛋白质展开过程的新知识可以作为对与分子拥挤,蛋白质聚集,原纤维形成以及通常在分子拥挤的局部环境下的基因翻译调控过程相关的人类疾病的机理的机械理解的一步。
更新日期:2018-06-27
中文翻译:

拥挤诱导的蛋白质自发变性和拥挤扰动的展开-折叠构象涨落动力学的单分子光谱研究
分子拥挤对蛋白质折叠-展开过程的影响对于了解活细胞中蛋白质的功能和结构动力学非常重要。在分子拥挤的情况下,由于熵作用降低而导致的蛋白质稳定性增强在实验和理论上都是众所周知的。然而,由于在蛋白质折叠和展开构象动力学的同样有利的环境中存在的各种相互作用之间的复杂性和相互作用,因此这种简单的减少的熵增强模型不足以描述蛋白质拥挤条件下的蛋白质折叠构象动力学。在本文中,我们报告了关于单个蛋白质分子自发地变性为未折叠蛋白质以及在拥挤试剂Ficoll 70溶液中折叠-展开的波动的观察结果。我们已经确定,这种蛋白质动力学涉及聚合物-聚合物相互作用,熵效应和蛋白质溶剂化的联合机制。动力学。我们通过使用单分子光谱学来表征蛋白质折叠-展开动力学,以获取有关蛋白质折叠-展开构象动力学的详细分子动力学尺度信息。我们的发现表明,复杂的展开动态过程是由分子拥挤效应诱导的单个蛋白质分子的自发变性,这在整体平均测量中难以捉摸。此外,自发展开所需的能量处于生物可及的力量波动水平,这暗示着人类健康的重要性和重要性。不均一的蛋白质展开过程的新知识可以作为对与分子拥挤,蛋白质聚集,原纤维形成以及通常在分子拥挤的局部环境下的基因翻译调控过程相关的人类疾病的机理的机械理解的一步。











































 京公网安备 11010802027423号
京公网安备 11010802027423号