当前位置:
X-MOL 学术
›
Bioconjugate Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Nature-Inspired Bioorthogonal Reaction: Development of β-Caryophyllene as a Chemical Reporter in Tetrazine Ligation
Bioconjugate Chemistry ( IF 4.0 ) Pub Date : 2018-05-31 00:00:00 , DOI: 10.1021/acs.bioconjchem.8b00283 Yunfei Wu 1, 2 , Jiulong Hu 1, 3 , Chen Sun 3 , Yu Cao 3 , Yuanhe Li 1 , Fayang Xie 1 , Tianyin Zeng 1 , Bing Zhou 3 , Juanjuan Du 1 , Yefeng Tang 1, 2
Bioconjugate Chemistry ( IF 4.0 ) Pub Date : 2018-05-31 00:00:00 , DOI: 10.1021/acs.bioconjchem.8b00283 Yunfei Wu 1, 2 , Jiulong Hu 1, 3 , Chen Sun 3 , Yu Cao 3 , Yuanhe Li 1 , Fayang Xie 1 , Tianyin Zeng 1 , Bing Zhou 3 , Juanjuan Du 1 , Yefeng Tang 1, 2
Affiliation
|
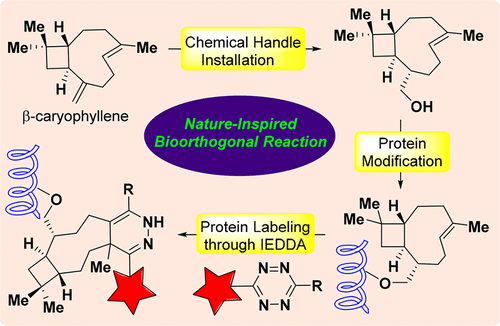
A nature-inspired bioorthogonal reaction has been developed, hinging on an inverse-electron-demand Diels–Alder reaction of tetrazine with β-caryophyllene. Readily accessible from the cheap starting material through a scalable synthesis, the newly developed β-caryophyllene chemical reporter displays appealing reaction kinetics and excellent biocompatibility, which renders it applicable to both in vitro protein labeling and live cell imaging. Moreover, it can be used orthogonally to the strain-promoted alkyne–azide cycloaddition for dual protein labeling. This work not only provides an alternative to the existing bioorthogonal reaction toolbox, but also opens a new avenue to utilize naturally occurring scaffolds as bioorthogonal chemical reporters.
中文翻译:

受自然启发的生物正交反应:在四嗪结扎反应中作为化学报告分子的β-石竹烯的开发。
已经开发出一种受自然启发的生物正交反应,它依赖于四嗪与β-石竹烯的电子逆需求Diels-Alder反应。新开发的β-石竹烯化学报告分子可通过廉价的起始原料通过可扩展的合成方法轻松获得,显示出诱人的反应动力学和出色的生物相容性,使其可用于体外蛋白质标记和活细胞成像。而且,它可以与应变促进炔烃-叠氮化物环加成反应正交使用,以进行双重蛋白质标记。这项工作不仅为现有的生物正交反应工具箱提供了替代方案,而且为利用天然存在的支架作为生物正交化学报告基因开辟了一条新途径。
更新日期:2018-05-31
中文翻译:

受自然启发的生物正交反应:在四嗪结扎反应中作为化学报告分子的β-石竹烯的开发。
已经开发出一种受自然启发的生物正交反应,它依赖于四嗪与β-石竹烯的电子逆需求Diels-Alder反应。新开发的β-石竹烯化学报告分子可通过廉价的起始原料通过可扩展的合成方法轻松获得,显示出诱人的反应动力学和出色的生物相容性,使其可用于体外蛋白质标记和活细胞成像。而且,它可以与应变促进炔烃-叠氮化物环加成反应正交使用,以进行双重蛋白质标记。这项工作不仅为现有的生物正交反应工具箱提供了替代方案,而且为利用天然存在的支架作为生物正交化学报告基因开辟了一条新途径。











































 京公网安备 11010802027423号
京公网安备 11010802027423号