当前位置:
X-MOL 学术
›
Org. Chem. Front.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Stereoselective allylic reduction via one-pot palladium-catalyzed allylic sulfonation and sulfinyl retro-ene reactions†
Organic Chemistry Frontiers ( IF 4.6 ) Pub Date : 2018-05-31 00:00:00 , DOI: 10.1039/c8qo00233a Hyun-Suk Um 1, 2, 3, 4 , Jungki Min 1, 2, 3, 4 , Taeyang An 1, 2, 3, 4 , Jin Choi 4, 5, 6, 7 , Chulbom Lee 1, 2, 3, 4
Organic Chemistry Frontiers ( IF 4.6 ) Pub Date : 2018-05-31 00:00:00 , DOI: 10.1039/c8qo00233a Hyun-Suk Um 1, 2, 3, 4 , Jungki Min 1, 2, 3, 4 , Taeyang An 1, 2, 3, 4 , Jin Choi 4, 5, 6, 7 , Chulbom Lee 1, 2, 3, 4
Affiliation
|
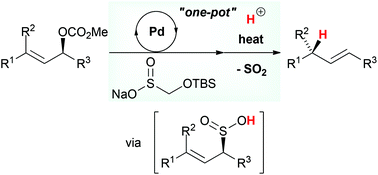
A tandem approach for the stereoselective allylic reduction has been developed based on a strategy combining the palladium-catalyzed S-allylation and the sulfinyl retro-ene reactions. In a one-pot process employing various allylic carbonates, sodium t-butyldimethylsilyloxymethanesulfinate (TBSOMS-Na), easily prepared from the commercially available reagent Rongalite™, serves as a potent nucleophile to engage in the Tsuji–Trost S-allylation reaction generating allylic sulfone products. Subsequently, in situ deprotection of the TBSOMS group reveals allylic sulfinic acids which undergo stereospecific reductive transposition via sulfur dioxide extrusion to provide alkene products.
中文翻译:

通过一锅钯催化的烯丙基磺化和亚磺酰基逆烯反应进行 立体选择性烯丙基还原†
基于结合钯催化的S-烯丙基化和亚磺酰基逆烯反应的策略,已经开发了用于立体选择的烯丙基还原的串联方法。在使用多种烯丙基碳酸酯的一锅法中,可从市售试剂Rongalite™轻松制备的叔丁基二甲基甲硅烷基氧基甲烷亚磺酸钠(TBSOMS-Na)作为有效的亲核试剂,参与Tsuji-Trost S-烯丙基化反应,生成烯丙基砜产品。随后,TBSOMS基团的原位脱保护揭示了烯丙基亚磺酸,其通过二氧化硫挤出经历立体有择的还原性转座以提供烯烃产物。
更新日期:2018-05-31
中文翻译:

通过一锅钯催化的烯丙基磺化和亚磺酰基逆烯反应进行 立体选择性烯丙基还原†
基于结合钯催化的S-烯丙基化和亚磺酰基逆烯反应的策略,已经开发了用于立体选择的烯丙基还原的串联方法。在使用多种烯丙基碳酸酯的一锅法中,可从市售试剂Rongalite™轻松制备的叔丁基二甲基甲硅烷基氧基甲烷亚磺酸钠(TBSOMS-Na)作为有效的亲核试剂,参与Tsuji-Trost S-烯丙基化反应,生成烯丙基砜产品。随后,TBSOMS基团的原位脱保护揭示了烯丙基亚磺酸,其通过二氧化硫挤出经历立体有择的还原性转座以提供烯烃产物。











































 京公网安备 11010802027423号
京公网安备 11010802027423号