当前位置:
X-MOL 学术
›
J. Fluorine Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Highly regioselective 4-hydroxy-1-methylpiperidine mediated aromatic nucleophilic substitution on a perfluorinated phthalimide core
Journal of Fluorine Chemistry ( IF 1.7 ) Pub Date : 2018-05-28 , DOI: 10.1016/j.jfluchem.2018.05.011 Ramóna Madácsi , Márió Gyuris , János Wölfling , László G. Puskás , Iván Kanizsai
中文翻译:

全氟邻苯二甲酰亚胺核心上的高度区域选择性的4-羟基-1-甲基哌啶介导的芳香亲核取代
更新日期:2018-05-28
Journal of Fluorine Chemistry ( IF 1.7 ) Pub Date : 2018-05-28 , DOI: 10.1016/j.jfluchem.2018.05.011 Ramóna Madácsi , Márió Gyuris , János Wölfling , László G. Puskás , Iván Kanizsai
|
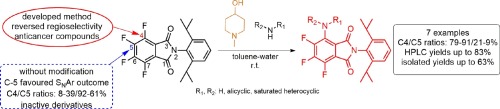
A tertiary amine-mediated highly regioselective aromatic nucleophilic substitution (SNAr) protocol was developed in the assemblies of perfluorinated phtalimide with primary or secondary amines as inputs. Application of 1-methyl-4-hydroxypiperidine as additive, formation of the less favoured, bioactive regioisomer was facilitated, modifying their ratios from the initial 8–36% to 81–91%. After optimization, a facile gram scale syntheses were accomplished and isolated the desired analogues in up to 63% yield.
中文翻译:

全氟邻苯二甲酰亚胺核心上的高度区域选择性的4-羟基-1-甲基哌啶介导的芳香亲核取代
在以伯胺或仲胺为输入的全氟化邻苯二甲酰亚胺的组装物中,开发了叔胺介导的高度区域选择性芳族亲核取代(S N Ar)方案。1-甲基-4-羟基哌啶作为添加剂的应用,促进了较不受欢迎的生物活性区域异构体的形成,将其比例从最初的8–36%更改为81–91%。优化后,完成了简便的克级合成,并以高达63%的收率分离了所需的类似物。











































 京公网安备 11010802027423号
京公网安备 11010802027423号