当前位置:
X-MOL 学术
›
Chem. Sci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
A paramedic treatment for modeling explicitly solvated chemical reaction mechanisms†
Chemical Science ( IF 7.6 ) Pub Date : 2018-05-30 00:00:00 , DOI: 10.1039/c8sc01424h Yasemin Basdogan 1, 2, 3, 4 , John A. Keith 1, 2, 3, 4
Chemical Science ( IF 7.6 ) Pub Date : 2018-05-30 00:00:00 , DOI: 10.1039/c8sc01424h Yasemin Basdogan 1, 2, 3, 4 , John A. Keith 1, 2, 3, 4
Affiliation
|
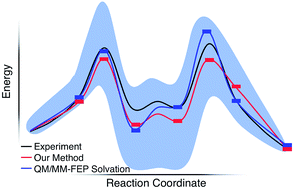
We report a static quantum chemistry modeling treatment to study how solvent molecules affect chemical reaction mechanisms without dynamics simulations. This modeling scheme uses a global optimization procedure to identify low energy intermediate states with different numbers of explicit solvent molecules and then the growing string method to locate sequential transition states along a reaction pathway. Testing this approach on the acid-catalyzed Morita–Baylis–Hillman (MBH) reaction in methanol, we found a reaction mechanism that is consistent with both recent experiments and computationally intensive dynamics simulations with explicit solvation. In doing so, we explain unphysical pitfalls that obfuscate computational modeling that uses microsolvated reaction intermediates. This new paramedic approach can promisingly capture essential physical chemistry of the complicated and multistep MBH reaction mechanism, and the energy profiles found with this model appear reasonably insensitive to the level of theory used for energy calculations. Thus, it should be a useful and computationally cost-effective approach for modeling solvent mediated reaction mechanisms when dynamics simulations are not possible.
中文翻译:

用于模拟显式溶剂化化学反应机理的护理人员治疗†
我们报告了静态量子化学建模处理,以研究溶剂分子如何在没有动力学模拟的情况下影响化学反应机理。该建模方案使用全局优化程序来识别具有不同数量的显式溶剂分子的低能中间状态,然后使用增长字符串方法沿反应路径定位顺序过渡状态。通过对甲醇中酸催化的森田-贝利斯-希尔曼(MBH)反应进行测试,我们发现了一种反应机理,该机理与最近的实验以及具有明显溶剂化的计算密集型动力学模拟均相一致。通过这样做,我们解释了非物理的陷阱,这些陷阱使使用微溶剂化反应中间体的计算模型难以理解。这种新的护理人员方法有望有望捕获复杂的多步MBH反应机理的基本物理化学,并且通过该模型发现的能量分布对于能量计算所用的理论水平似乎相当不敏感。因此,当无法进行动力学模拟时,它应该是一种有用的且具有计算成本效益的方法,用于对溶剂介导的反应机理进行建模。
更新日期:2018-05-30
中文翻译:

用于模拟显式溶剂化化学反应机理的护理人员治疗†
我们报告了静态量子化学建模处理,以研究溶剂分子如何在没有动力学模拟的情况下影响化学反应机理。该建模方案使用全局优化程序来识别具有不同数量的显式溶剂分子的低能中间状态,然后使用增长字符串方法沿反应路径定位顺序过渡状态。通过对甲醇中酸催化的森田-贝利斯-希尔曼(MBH)反应进行测试,我们发现了一种反应机理,该机理与最近的实验以及具有明显溶剂化的计算密集型动力学模拟均相一致。通过这样做,我们解释了非物理的陷阱,这些陷阱使使用微溶剂化反应中间体的计算模型难以理解。这种新的护理人员方法有望有望捕获复杂的多步MBH反应机理的基本物理化学,并且通过该模型发现的能量分布对于能量计算所用的理论水平似乎相当不敏感。因此,当无法进行动力学模拟时,它应该是一种有用的且具有计算成本效益的方法,用于对溶剂介导的反应机理进行建模。











































 京公网安备 11010802027423号
京公网安备 11010802027423号