Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Potential enthalpic energy of water in oils exploited to control supramolecular structure
Nature ( IF 50.5 ) Pub Date : 2018-05-30 , DOI: 10.1038/s41586-018-0169-0 Nathan J. Van Zee , Beatrice Adelizzi , Mathijs F. J. Mabesoone , Xiao Meng , Antonio Aloi , R. Helen Zha , Martin Lutz , Ivo A. W. Filot , Anja R. A. Palmans , E. W. Meijer
Nature ( IF 50.5 ) Pub Date : 2018-05-30 , DOI: 10.1038/s41586-018-0169-0 Nathan J. Van Zee , Beatrice Adelizzi , Mathijs F. J. Mabesoone , Xiao Meng , Antonio Aloi , R. Helen Zha , Martin Lutz , Ivo A. W. Filot , Anja R. A. Palmans , E. W. Meijer
|
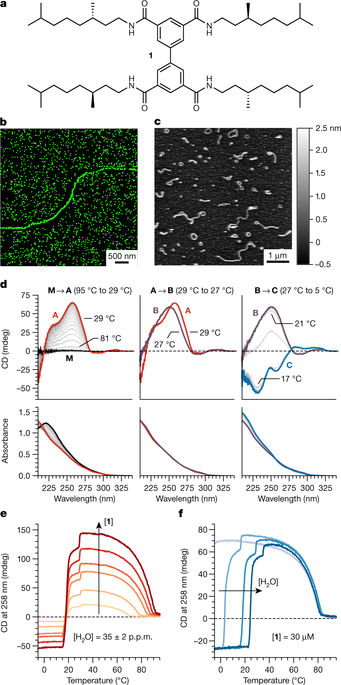
Water directs the self-assembly of both natural1,2 and synthetic3–9 molecules to form precise yet dynamic structures. Nevertheless, our molecular understanding of the role of water in such systems is incomplete, which represents a fundamental constraint in the development of supramolecular materials for use in biomaterials, nanoelectronics and catalysis10. In particular, despite the widespread use of alkanes as solvents in supramolecular chemistry11,12, the role of water in the formation of aggregates in oils is not clear, probably because water is only sparingly miscible in these solvents—typical alkanes contain less than 0.01 per cent water by weight at room temperature13. A notable and unused feature of this water is that it is essentially monomeric14. It has been determined previously15 that the free energy cost of forming a cavity in alkanes that is large enough for a water molecule is only just compensated by its interaction with the interior of the cavity; this cost is therefore too high to accommodate clusters of water. As such, water molecules in alkanes possess potential enthalpic energy in the form of unrealized hydrogen bonds. Here we report that this energy is a thermodynamic driving force for water molecules to interact with co-dissolved hydrogen-bond-based aggregates in oils. By using a combination of spectroscopic, calorimetric, light-scattering and theoretical techniques, we demonstrate that this interaction can be exploited to modulate the structure of one-dimensional supramolecular polymers.Less than 0.01 per cent by weight of water in an alkane solvent is sufficient to interact with co-dissolved supramolecular polymeric chains by hydrogen bonding and modulate the structure of the assembly.
更新日期:2018-05-30











































 京公网安备 11010802027423号
京公网安备 11010802027423号