Electrochemistry Communications ( IF 5.4 ) Pub Date : 2018-05-30 , DOI: 10.1016/j.elecom.2018.05.026 Benchaporn Lertanantawong , Panjaphong Lertsathitphong , Anthony P. O'Mullane
|
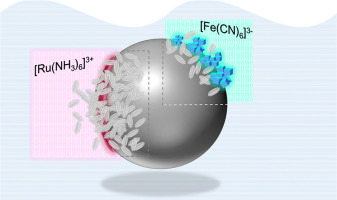
Room temperature liquid metals based on gallium demonstrate interesting physical and chemical properties when placed in electrochemical environments. In this work we explore the applicability of hanging liquid metal droplets as electrode materials for simple surface insensitive and surface sensitive electron transfer reactions, namely the electrochemical reduction of [Ru(NH3)6]3+ and [Fe(CN)6]3− ions in solution. Significantly we found that for both redox species the electron transfer process was impeded at galinstan (68.5% Ga, 22.5% In and 10% Sn). This is related to the chemical reactivity of the Ga component which is oxidised when exposed to aqueous solutions of [Ru(NH3)6]3+ and [Fe(CN)6]3− to generate the reduced form of the redox mediator. For the former this results in the production of Ru-red and Ru-brown cationic trimers in solution as well as gallium oxide on the surface of the liquid metal, and in the latter case the formation of solid Prussian Blue.
中文翻译:

Ga基液态金属与氧化还原活性物质的化学反应性及其对电化学过程的影响
当置于电化学环境中时,基于镓的室温液态金属表现出令人感兴趣的物理和化学性质。在这项工作中,我们探讨了悬挂金属液滴作为电极材料对简单的表面不敏感和表面敏感的电子转移反应(即[Ru(NH 3)6 ] 3+和[Fe(CN)6 ] 3的电化学还原)的适用性−溶液中的离子。显着地,我们发现对于这两种氧化还原物种,加林斯坦(68.5%的Ga,22.5%的In和10%的Sn)都阻碍了电子转移过程。这与暴露于[Ru(NH 3)的水溶液中时被氧化的Ga组分的化学反应性有关6 ] 3+和[Fe(CN)6 ] 3-生成还原形式的氧化还原介体。对于前者,这会导致在溶液中生成Ru-红色和Ru-棕色阳离子三聚体,以及在液态金属表面上生成氧化镓,而在后者的情况下,则会生成固体普鲁士蓝。



























 京公网安备 11010802027423号
京公网安备 11010802027423号